Philadelphia, December 13, 2021 — Even after the Food and Drug Administration (FDA) issued a safety notice and recall, manufacturing defects in certain cardiac rhythm management (CRM) devices led to premature battery depletion that was not picked up by remote monitoring. A case report in Heart Rhythm Case Reports, an official journal of the Heart Rhythm Society, published by Elsevier, documents two instances of premature battery failures in a recalled subset of St. Jude Assurity and Endurity pacemakers (manufactured by Abbott) that shed light on a potentially lethal flaw of remote monitoring.
Credit: Heart Rhythm Case Reports
Philadelphia, December 13, 2021 — Even after the Food and Drug Administration (FDA) issued a safety notice and recall, manufacturing defects in certain cardiac rhythm management (CRM) devices led to premature battery depletion that was not picked up by remote monitoring. A case report in Heart Rhythm Case Reports, an official journal of the Heart Rhythm Society, published by Elsevier, documents two instances of premature battery failures in a recalled subset of St. Jude Assurity and Endurity pacemakers (manufactured by Abbott) that shed light on a potentially lethal flaw of remote monitoring.
The case report highlights how the device maker’s recommendations for managing affected patients post recall did not account for this shortcoming or sufficiently address patient safety, even with the FDA’s repeated actions. The initial recommendations were to ensure standard monitoring was in place, which consists of manual patient-initiated transmissions every three months and pacemaker-initiated alerts for certain device-related issues.
“The cases we present highlight the limitations of remote monitoring for the early identification of CRM device battery failure, particularly in cases in which sudden complete battery failure is possible,” said author Michael J. Cutler, DO, PhD, Intermountain Heart Institute, Murray, Utah, USA. “As such, current manufacturer recommendations for reliance on remote monitoring for early detection of premature battery depletion are likely not sufficient for pacemaker-dependent patients.”
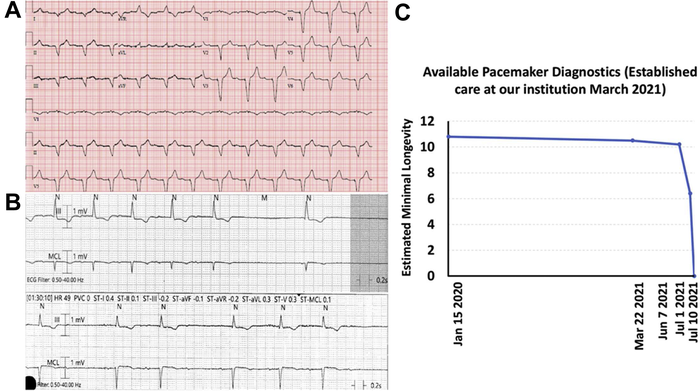
The FDA issued a safety notice about a subset of St. Jude Assurity and Endurity pacemakers on March 15, 2021, with an initial recommendation for remote monitoring through Merlin.net, which provides an automated alert within 24 hours of a device reaching Elective Replacement Indicator and End of Service. The FDA subsequently upgraded its advisory to a Class I recall of a larger cohort of these pacemakers, with recommendations for closer remote monitoring of implanted devices – advice that the evidence suggests might not detect all failures.
The two patients presented in the case study experienced unexpected and complete loss of pacing via their CRM devices due to complete battery depletion, resulting in symptoms requiring urgent device replacement. In both cases there was no communication between the pacemaker generator and the Merlin communicator because of abrupt battery failure that subsequently caused a loss of pacing; as a result, no alert was sent to their physicians. Flawed preparation of the epoxy used in manufacturing allowed moisture to seep into some devices and cause premature battery depletion and loss of pacing without forewarning. Loss of transmitting capability from a pacemaker may not be immediately detected and the resulting loss of all pacing functions may go undetected by remote monitoring.
Because these two cases occurred around the same time and in close geographic proximity, it raises the possibility that there are significantly more cases than initially suspected, and that Abbott’s estimate of a 0.01% failure rate for 335,000 potentially affected active devices worldwide represents only a fraction of actual cases. Many more pacemakers involved in the recall may have already or will abruptly lose their ability to pace. Continued surveillance and FDA reporting of relevant adverse events are essential for these devices to establish the incidence and frequency of these events and guide recommendations in the future.
Dr. Cutler explained that the cases were submitted for publication “to inform and alert physicians treating affected patients of the possibility of an abrupt loss-of-pacing failure, with potentially life-threatening implications. Implanting physicians and practices that manage patients with these devices should be aware of this possible failure mode. Consideration should be given to close monitoring or to prophylactic generator change in appropriate patients.”
He further noted that his team has elected the latter option, evaluating patients with recalled devices on a case-by-case basis to consider changing out the pacemaker in patients for whom a loss-of-pacing failure may be life threatening. “The process of developing this course of action highlights the opportunity for improved collaboration between industry, health systems, medical societies, and individual healthcare providers in the management of CRM device performance deficiencies.”
Furthermore, since the initial patient cases were reported to Abbott, the manufacturer has developed a software patch called “EPI,” which allows closer monitoring of battery life for the devices affected by this recall. This software may obviate the need for prophylactic generator changes in many patients.
“The software patch is an important tool in monitoring our patients and may be able to pick up most cases of premature battery depletion. We suggest physicians discuss the role of using the software as an alternative to generator changeout in appropriate patients. Abbott developed the software in response to our concerns, and this reflects the importance of the role of treating physicians in working with industry to ensure that our patients get optimal care,” added lead author of the paper Yonathan F. Melman, MD, PhD, McKay Dee Medical Center, Intermountain Heart Institute, Ogden, UT, USA.
Writing in an accompanying editorial, Mikhael F. El-Chami, MD, Professor of Medicine, Emory University School of Medicine, Atlanta, GA, USA, commented that the authors of this case report should be commended for reporting these two cases. “When it comes to identifying problems with cardiac implantable electronic devices, the onus is not only on the device manufacturer and the FDA but also on the physicians. The latter are on the forefront and are often the first to encounter problems with these devices. Reporting these potential malfunctions will help regulatory agencies and device manufacturers identify whether these issues fall within the expected range of a malfunction or exceed it. Therefore, complacency should have no role when it comes to identifying and reporting unexpected device malfunctions.”
Journal
HeartRhythm Case Reports
DOI
10.1016/j.hrcr.2021.09.013
Method of Research
Case study
Subject of Research
People
Article Title
Limitations of manufacturer-recommended remote monitoring in the St. Jude Assurity/Endurity battery recall




