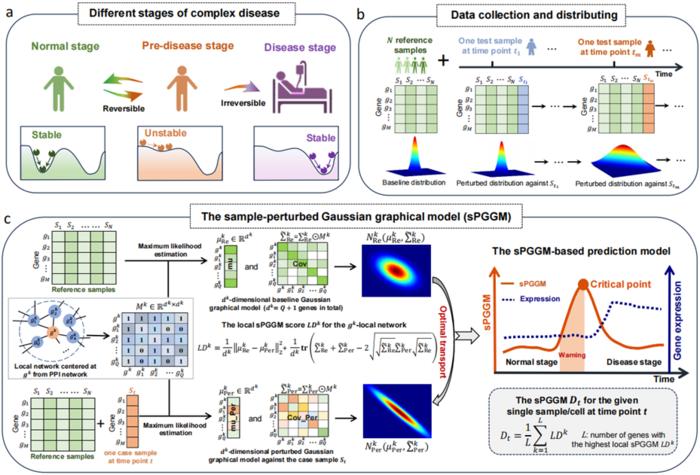
In an innovative approach to deciphering the complexities of disease progression, researchers have developed a groundbreaking method known as the sample-perturbed Gaussian graphical model (sPGGM). This model aims to identify pre-disease stages by analyzing dynamic shifts in biological systems that precede the manifestation of symptoms. As diseases evolve, they often traverse through multiple phases that can be critical for timely diagnosis and intervention. The research is spearheaded by leading mathematicians Prof. Rui Liu and Prof. Pei Chen from the South China University of Technology, alongside Dr. Jiayuan Zhong from Foshan University, and their findings stand to revolutionize our understanding of disease mechanisms.
Understanding disease dynamics is not merely an academic interest; it bears significant implications for patient care and treatment strategies. The disease progression can often be categorized into three distinct states: the normal stage, the pre-disease stage, and the disease stage. During the normal stage, individuals experience high stability and exhibit no symptoms of illness. However, as one approaches the pre-disease stage—which serves as a critical threshold—subtle internal or external disturbances can precipitate a drastic shift toward irreversible health deterioration. Recognizing these transitions is essential for early detection and preventive strategies, but it is fraught with challenges due to the nuanced differences between the normal and pre-disease phases.
.adsslot_TcS9Qn1BI0{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_TcS9Qn1BI0{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_TcS9Qn1BI0{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
The sPGGM employs optimal transport theory to delineate the differences in distributions between two sets of data: the baseline distribution, derived from reference samples, and a perturbed distribution, which integrates specific case samples. By fitting these distributions, researchers create a model that not only identifies potential shifts but quantifies them using the Wasserstein distance—a metric that measures how “far apart” two probability distributions are. This approach amplifies the model’s sensitivity to critical transitions, allowing it to capture dramatic changes often overlooked by conventional analysis.
To validate the efficacy of the sPGGM, the research team conducted rigorous testing on both simulated datasets and real-world disease datasets. Remarkably, they applied the model to six cancer datasets from the widely respected TCGA database, which encompasses a diverse range of carcinoma types, each presenting unique challenges in terms of detection and analysis. The robustness of the sPGGM was evidenced across various cancer types, showcasing its versatility and adaptability in clinical settings.
In practical applications, the sPGGM is positioned not just as a theoretical construct but as a tangible tool for clinicians and researchers alike. Its ability to discern pre-disease signals amid a backdrop of noise and variability empowers medical professionals to make more informed decisions regarding patient care. Moreover, the enhanced detection capabilities of the sPGGM translate into a more personalized approach to treatment, tailoring interventions based on individual patient profiles rather than a one-size-fits-all methodology.
What sets the sPGGM apart from existing single-sample detection methods is its unique approach to integrating prior biological knowledge. By embedding the Gaussian graphical model with data from established protein-protein interaction networks, the sPGGM constructs a framework that not only detects transition stages but extrapolates potential signaling molecules involved in disease progression. This fusion of computational modeling and biological knowledge represents a significant advancement in understanding complex interactions within biological systems.
Clinical implications of this research could be groundbreaking. The ability to pinpoint the pre-disease stage accurately holds the promise of intervening before symptoms arise, potentially mitigating the impact of various diseases. The key is in identifying those rare molecular signals that act as precursors to disease onset, and the research demonstrates a clear pathway to achieving this goal.
The results from the study advocate for a paradigm shift in how we approach disease monitoring and intervention. By prioritizing the identification of pre-disease markers, healthcare practitioners can employ preventive measures far more effectively than ever before. Ultimately, the goal is to redirect our focus from merely treating established diseases to preventing their onset through early detection.
The success of the sPGGM has far-reaching implications not only for oncology but also for the broader field of medicine. Its principles can be adapted to tackle various chronic diseases, thereby enhancing our overall capacity to manage public health concerns. Furthermore, as the biomedical field increasingly relies on big data and complex analyses, methodologies like sPGGM will become indispensable tools for bridging the gap between raw data and actionable insights.
In closing, the introduction of the sample-perturbed Gaussian graphical model represents a significant milestone in the quest to enhance disease detection and intervention. By addressing the intricacies of disease progression through a mathematical and computational lens, researchers have opened up new avenues for understanding and ultimately combating complex diseases. This pioneering work is not just an academic exercise; it signifies a major leap toward more personalized healthcare solutions that can profoundly impact patient outcomes.
Subject of Research: Detection of pre-disease stages in disease progression
Article Title: Innovative Method for Early Detection of Disease Stages
News Publication Date: October 2023
Web References: http://dx.doi.org/10.1093/nsr/nwaf189
References: National Science Review
Image Credits: ©Science China Press
Keywords
Disease progression, pre-disease stage, sample-perturbed Gaussian graphical model, optimal transport theory, cancer detection, personalized medicine, gene expression, protein-protein interaction networks, Wasserstein distance, early intervention.
Tags: biological system dynamicscritical thresholds in healthdisease mechanism understandingdisease progression analysisearly disease detection methodshealth stability phasesinnovative disease diagnosis techniquesintervention strategies in medicinemathematical modeling in healthcarepre-disease stage identificationsample-perturbed Gaussian graphical modelsignaling molecules in disease