In groundbreaking new research published in Nature Communications, scientists have uncovered a pivotal role for intestinal interleukin-22 (IL-22) in enhancing the production of glucagon-like peptide-1 (GLP-1), a key hormone involved in glucose regulation. This discovery opens promising avenues for the treatment of obesity-induced insulin resistance and type 2 diabetes. The study, conducted on male mice subjected to a high-fat diet, delineates a novel molecular mechanism whereby IL-22 acts through the STAT3 signaling pathway to significantly improve glucose homeostasis, representing a crucial breakthrough in metabolic disease research.
Obesity, particularly that driven by high-fat diets, is a major global health crisis linked to the development of insulin resistance, type 2 diabetes, and related metabolic syndromes. One of the critical hormonal regulators of glucose metabolism is GLP-1, a peptide produced by intestinal L-cells that stimulates insulin secretion and suppresses glucagon release, thereby lowering blood sugar levels. Enhancing GLP-1 levels has long been a target of pharmacological intervention, but the endogenous regulatory mechanisms governing its secretion remain incompletely understood.
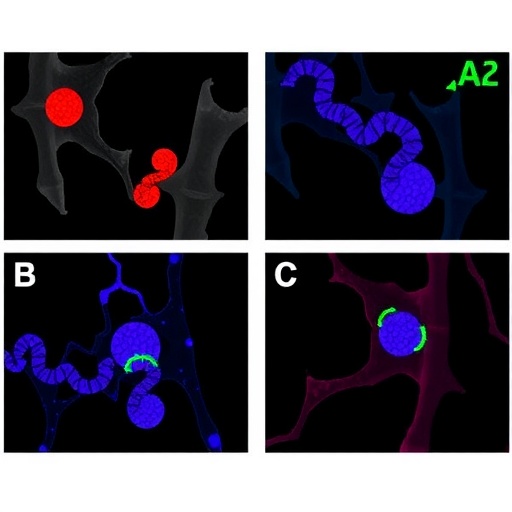
The team led by Kim, Ahn, and Lee has shed light on this complex biological interplay, demonstrating that intestinal IL-22, a cytokine primarily known for its role in immune function and tissue repair, can directly stimulate GLP-1 production. IL-22 exerts its effects by activating the STAT3 (signal transducer and activator of transcription 3) pathway within intestinal epithelial cells, which then promotes GLP-1 gene expression and secretion. This connection between immune signaling and metabolic regulation reveals an unexpected dimension of gut biology that may be exploited therapeutically.
In their experiments, the researchers utilized a well-established murine model of diet-induced obesity, whereby male mice were fed a high-fat diet to mimic the metabolic complications commonly seen in humans. They observed that levels of intestinal IL-22 were significantly correlated with GLP-1 secretion and improved glucose tolerance. Genetic and pharmacological interventions that increased IL-22 signaling led to a marked enhancement of GLP-1 levels, which in turn improved insulin sensitivity and glucose clearance.
Mechanistically, the STAT3 pathway was confirmed as the critical mediator between IL-22 receptor activation and GLP-1 gene transcription. Using selective inhibitors and genetic knockdowns, the researchers were able to demonstrate that disrupting STAT3 signaling abrogated the beneficial metabolic effects of IL-22. This highlights STAT3 as a potential molecular target for drug development aimed at mimicking or amplifying the endogenous effects of IL-22 on glucose metabolism.
Beyond the mechanistic insights, the study importantly establishes IL-22 as a physiologically relevant factor in metabolic regulation during obesity. Chronic high-fat feeding typically suppresses many beneficial gut hormones and exacerbates insulin resistance. However, IL-22 appears to counteract these detrimental effects by sustaining GLP-1 production and maintaining glucose homeostasis. This dual role of IL-22 in immune defense and metabolic control underscores the emerging concept of the gut as an immunometabolic organ.
Further investigations revealed that administration of recombinant IL-22 to obese mice not only boosted GLP-1 secretion but also led to improved overall metabolic profiles, including reduced fasting glucose levels and better insulin responsiveness. These results suggest that IL-22 or agents that induce its production might serve as innovative therapies to treat or prevent type 2 diabetes, particularly in the context of diet-induced obesity.
The implications of this study extend well beyond murine models. Given the evolutionary conservation of IL-22 and GLP-1 biology, future clinical research may validate IL-22–STAT3 modulation as an effective strategy for metabolic diseases in humans. Moreover, understanding how obesity and diet impact IL-22 expression could identify new predictive biomarkers for metabolic health and diabetes risk.
This research also intersects intriguingly with the burgeoning field of microbiome-host interactions. IL-22 production by intestinal immune cells is often influenced by microbial metabolites and luminal cues. Hence, the gut microbiota may indirectly regulate glucose homeostasis by modulating IL-22 dynamics, linking diet, immunity, and metabolism in a complex biological web.
It is also notable that IL-22 has been extensively studied for its role in maintaining intestinal barrier integrity. This new evidence connecting IL-22 to GLP-1 secretion adds another layer to its multifaceted contributions to gut physiology, positioning IL-22 as a master regulator of both immune defense and metabolic function. These dual roles make IL-22 a highly attractive candidate for multifactorial intervention strategies targeting obesity-related diseases.
Despite its promise, the translation of these findings into clinical practice will require careful consideration of the pleiotropic effects of IL-22 signaling. Because IL-22 has powerful effects on immune pathways, systemic manipulation may carry risks of inflammation or dysregulated immune responses. Future research must therefore focus on tissue-specific delivery methods or targeted modulation of downstream signaling components like STAT3 to ensure safety.
Intriguingly, this study opens the door to exploring IL-22 analogs or small molecules capable of selectively enhancing the IL-22–STAT3–GLP-1 axis in the gut. Such therapies could complement existing GLP-1 receptor agonists, potentially offering enhanced efficacy through endogenous hormone upregulation rather than receptor stimulation alone. The synergy of immunological and metabolic therapeutics represents a tantalizing frontier in biomedical innovation.
In summary, Kim and colleagues’ discovery that intestinal IL-22 enhances GLP-1 production via the STAT3 pathway profoundly enriches our understanding of metabolic regulation during high-fat diet-induced obesity. The detailed molecular insights and robust in vivo validation provide a compelling narrative linking immunity, endocrinology, and metabolism. This synergy between immune cytokines and incretin biology could catalyze novel therapeutic approaches poised to tackle the global diabetes epidemic.
As the field advances, integrating the IL-22–STAT3 pathway into broader frameworks of gut-liver-pancreas axis communication could unravel even more intricate mechanisms influencing metabolic health. The convergence of diet, microbiota, immune function, and hormone secretion illustrated here exemplifies the complexity of systemic homeostasis and highlights the gut as a central orchestrator.
This landmark research not only elucidates new biology but also offers a beacon of hope for millions affected by obesity and diabetes. By harnessing the body’s endogenous cytokine networks, future therapies might restore metabolic balance with enhanced precision, reduced side effects, and long-lasting efficacy. The promise of IL-22-driven GLP-1 enhancement is indeed a paradigm-shifting stride toward conquering metabolic disease.
Subject of Research: The role of intestinal interleukin-22 in enhancing GLP-1 production through the STAT3 pathway to improve glucose homeostasis during high-fat diet–induced obesity.
Article Title: Intestinal interleukin-22 enhances GLP-1 production via the STAT3 pathway to improve glucose homeostasis during high-fat diet induced obesity in a study with male mice.
Article References: Kim, CW., Ahn, JH., Lee, B.R. et al. Intestinal interleukin-22 enhances GLP-1 production via the STAT3 pathway to improve glucose homeostasis during high-fat diet induced obesity in a study with male mice. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69734-0
Image Credits: AI Generated
Tags: cytokine regulation of metabolic diseasesendogenous GLP-1 modulationGLP-1 secretion enhancement mechanismshigh-fat diet impact on glucose homeostasisIL-22 role in glucose regulationIL-22 STAT3 signaling pathway in metabolismimmune function and metabolic health connectionintestinal interleukin-22 and glucagon-like peptide-1 interactionintestinal L-cell hormone productionmetabolic syndrome and inflammatory cytokinesobesity-induced insulin resistance treatmenttype 2 diabetes novel therapies