Researchers develop a method for on-demand hydrogen production, which has potential for use in portable hydrogen fuel cells
Credit: Jing Liu
WASHINGTON, January 28, 2020 — Since the Industrial Revolution, the environmental impacts of energy have posed a concern. Recently, this has driven researchers to search for viable options for clean and renewable energy sources.
Due to its affordability and environmental friendliness, hydrogen is a feasible alternative to fossil fuels for energy applications. However, due to its low density, hydrogen is difficult to transport efficiently, and many on-board hydrogen generation methods are slow and energy intensive.
Researchers from the Chinese Academy of Sciences, Beijing and Tsinghua University, Beijing investigate real-time, on-demand hydrogen generation for use in fuel cells, which are a quiet and clean form of energy. They describe their results in the Journal of Renewable and Sustainable Energy, from AIP Publishing.
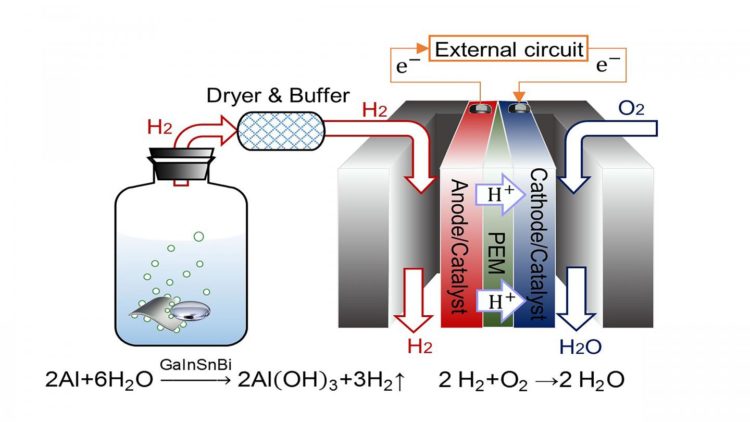
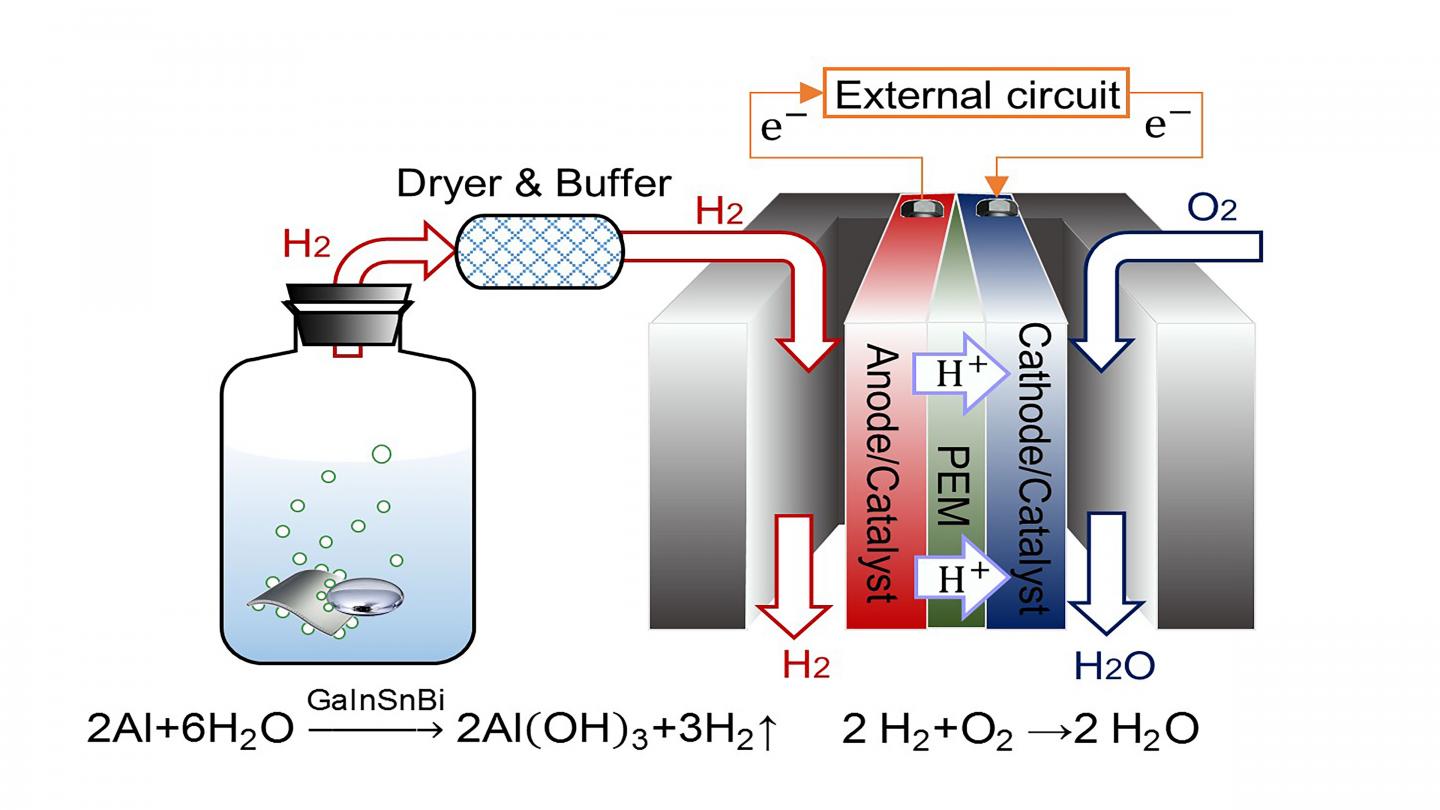
The researchers used an alloy — a combination of metals — of gallium, indium, tin and bismuth to generate hydrogen. When the alloy meets an aluminum plate immersed in water, hydrogen is produced. This hydrogen is connected to a proton exchange membrane fuel cell, a type of fuel cell where chemical energy is converted into electrical energy.
“Compared with traditional power generation methods, PEMFC inherits a higher conversion efficiency,” said author Jing Liu, a professor at the Chinese Academy of Sciences and Tsinghua University. “It could start rapidly and run quietly. Moreover, a key benefit to this process is that the only product it generates is water, making it environmentally friendly.”
They found the addition of bismuth to the alloy has a large effect on hydrogen generation. Compared to an alloy of gallium, indium and tin, the alloy including bismuth leads to a more stable and durable hydrogen generation reaction. However, it is important to be able to recycle the alloy in order to further reduce cost and environmental impact.
“There are various problems in existing methods for post-reaction mixture separation,” Liu said. “An acid or alkaline solution can dissolve aluminum hydroxide but also causes corrosion and pollution problems.”
Other byproduct removal methods are difficult and inefficient, and the problem of heat dissipation in the hydrogen reaction process also needs to be optimized. Once these difficulties are resolved, this technology can be used for applications from transportation to portable devices.
“The merit of this method is that it could realize real-time and on-demand hydrogen production,” said Liu. “It may offer a possibility for a green and sustainable energy era.”
###
The article, “Instant hydrogen production using Ga-In-Sn-Bi alloy-activated Al-water reaction for hydrogen fuel cells,” is authored by Shuo Xu, Yuntao Cui, Lixiang Yang and Jing Liu. The article will appear in Journal of Renewable and Sustainable Energy on Jan. 28, 2020 (DOI: 10.1063/1.5124371). After that date, it can be accessed at http://aip.
Media Contact
Larry Frum
[email protected]
301-209-3090
Related Journal Article
http://dx.