In a groundbreaking development within the realm of anti-inflammatory therapeutics, Insilico Medicine, a clinical-stage pioneer harnessing the power of generative artificial intelligence, has publicly announced the nomination of a novel small molecule inhibitor, ISM5059. Unlike traditional drug discovery paradigms, ISM5059 emerges from the synergy of advanced AI-driven design and meticulous preclinical validation, marking a significant stride in targeting systemic inflammation through a peripherally restricted mechanism aimed specifically at the NLRP3 inflammasome pathway.
The NOD-like receptor protein 3 (NLRP3) inflammasome has long stood as a pivotal molecular complex regulating innate immune responses. Upon activation by diverse internal or external stimuli, NLRP3 orchestrates the release of proinflammatory cytokines, notably Interleukin-1β (IL-1β) and Interleukin-18 (IL-18), acting as master regulators of the inflammatory cascade. This activation not only drives inflammation but precipitates pyroptosis—a highly inflammatory form of programmed cell death contributing to disease pathogenesis across a spectrum of metabolic, cardiovascular, and autoimmune disorders. As a validated therapeutic target, NLRP3 modulation offers profound potential; however, achieving selective inhibition with an optimized safety profile has remained a formidable challenge.
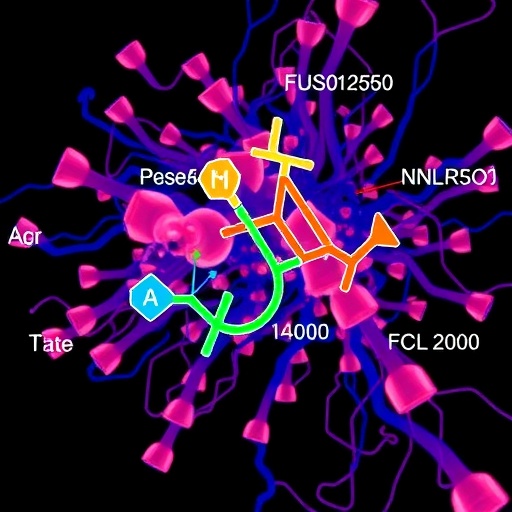
ISM5059’s distinctive success lies in its novel chemical core, deliberately engineered to impart high peripheral selectivity, which contrasts starkly with Insilico’s earlier brain-penetrant NLRP3 inhibitor, ISM8969. This design strategy aims to confine the compound’s pharmacodynamic effects outside the central nervous system, thereby minimizing potential neurotoxicity risks. Leveraging their proprietary Pharma.AI platform, Insilico Medicine meticulously generated ISM5059’s innovative molecular architecture to stabilize the inactive conformation of NLRP3 and obstruct its oligomerization — a critical step in the inflammasome assembly and subsequent cytokine liberation.
Preclinical evaluations of ISM5059 have demonstrated remarkable efficacy and safety. In vivo models, particularly the peritonitis model used to evaluate acute inflammatory responses, revealed a striking dose-dependent suppression of IL-1β release. Notably, even at the minimal dose of 0.3 mg/kg, ISM5059 succeeded in halving IL-1β levels relative to control groups. Higher doses elicited progressively robust inhibitory effects, underscoring ISM5059’s potent capacity to dampen acute systemic inflammation. Such compelling data presage considerable therapeutic benefits in managing inflammation-driven pathologies.
Beyond acute inflammation, the implications of NLRP3 blockade by ISM5059 extend to multifaceted chronic conditions where dysregulated inflammatory responses are pathognomonic. Metabolic disorders such as obesity, type 2 diabetes, and hyperlipidemia, as well as cardiovascular diseases, are all underpinned by sustained inflammasome activation. By curtailing this key upstream driver, ISM5059 holds promise for modulating disease progression and improving patient outcomes in these prevalent health crises that demand novel pharmacological solutions.
Another dimension of ISM5059’s attractiveness is its predicted low efficacious dose in humans. Through AI-guided optimization, the molecule exhibits a high safety margin, a critical consideration in systemic therapies targeting inflammation where long-term administration is often necessary. Early toxicity screening has revealed no indication of central nervous system adverse effects, further validating the peripherally restricted design that makes ISM5059 a potential front-runner in broad systemic inflammatory disease management.
This latest advancement is timely, complementing Insilico’s earlier FDA Investigational New Drug (IND) clearance of ISM8969 designed for neurodegenerative diseases such as Parkinson’s, where brain penetration is necessary. Together, these compounds exemplify Insilico’s dual-pronged strategy to create differentiated NLRP3 inhibitors tailored for distinct disease contexts—central nervous system versus peripheral organ systems—thus maximizing therapeutic reach while circumventing overlapping safety concerns.
The nimbleness of Insilico Medicine’s AI-powered drug discovery platform is also remarkable, with an average preclinical candidate nomination timeline remarkably compressed to 12-18 months—a fraction of the traditional multi-year timeline. This efficiency is coupled with a streamlined synthesis and testing process, involving dynamic iterations of merely 60 to 200 molecules per program. It sets a new benchmark for the pharmaceutical industry’s approach to innovative drug development, especially in complex targets like inflammasomes which traditionally risk protracted and costly attrition.
Looking to the future, Insilico Medicine plans to exploit ISM5059’s wide therapeutic index across a swath of indications extending beyond immunology and inflammation. Autoimmune disorders, certain ophthalmological conditions characterized by chronic inflammation, and cardiometabolic diseases present compelling arenas for ISM5059’s clinical exploration. Such diversity in applicability is a testament to the inflammasome’s centrality in pathological inflammation and the molecule’s versatile pharmacological profile.
The implications of successfully targeting NLRP3 with a molecule like ISM5059 extend beyond mere inflammation suppression. By intercepting this pathway at its inception, there lies the potential to mitigate maladaptive immune responses before irreversible tissue damage ensues. This preventative approach resonates with the modern paradigm shift toward precision medicine, offering tailored, mechanism-based therapies that yield efficacy without compromising safety — a crucial balance in chronic disease management.
In summary, Insilico Medicine’s introduction of ISM5059 as a preclinical candidate embodies a milestone in AI-augmented drug discovery, marrying innovative chemistry with biological insight and computational prowess. The inhibitor’s high peripheral selectivity, profound potency, and favorable safety profile crystallize the promise of next-generation inflammasome therapies. As ISM5059 moves toward clinical development, it represents a beacon of hope for patients suffering from systemic inflammatory diseases desperately in need of safer, more effective treatments.
Subject of Research: The research focuses on ISM5059, a novel peripherally restricted small molecule inhibitor targeting the NLRP3 inflammasome, designed for systemic inflammatory diseases including autoimmune, metabolic, and cardiovascular disorders.
Article Title: Insilico Medicine’s ISM5059: A Generative AI-Designed Peripheral NLRP3 Inhibitor Poised to Revolutionize Systemic Inflammatory Disease Treatment
News Publication Date: Not specified explicitly in the given content.
Web References: www.insilico.com
Image Credits: Insilico Medicine
Keywords: Generative AI, NLRP3 inflammasome, IL-1β, inflammation, systemic inflammatory diseases, drug discovery, preclinical candidate, peripheral restriction, autoimmune diseases, metabolic disorders, cardiovascular diseases, pharmacology
Tags: AI-driven drug discoveryanti-inflammatory therapeuticscytokine release regulationinnovative small molecule inhibitorsInsilico MedicineISM5059 NLRP3 inhibitormetabolic and autoimmune disordersNLRP3 inflammasome pathwayperipheral selectivity in drugspreclinical candidate selectionprogrammed cell death pyroptosistherapeutic target modulation