In an era defined by rapid advancements in material science and sensing technologies, a groundbreaking discovery in the realm of charge transfer chemistry promises to revolutionize the way we detect environmental pollutants. Researchers at the Shibaura Institute of Technology (SIT) in Japan have developed a novel molecular system that exhibits a remarkable, reversible color change upon interaction with naphthalene—a common yet environmentally regulated hydrocarbon. This breakthrough hinges on the delicate interplay between intramolecular and intermolecular charge transfers within a uniquely designed pyrazinacene derivative, opening exciting new possibilities for highly sensitive and selective colorimetric sensors.
Charge transfer (CT) phenomena involve the movement of electrons either within a single molecule or between molecules. These electron transfers can profoundly influence the optical and electronic properties of materials, rendering them invaluable in various applications from organic electronics to photovoltaic devices. Intramolecular charge transfer (ICT) refers specifically to electron migration between donor and acceptor groups embedded within the same molecular framework. This effect can cause a noticeable redshift in absorption spectra, a principle exploited in the development of dyes and organic light-emitting diodes (OLEDs). Conversely, intermolecular CT occurs when electrons traverse from donor to acceptor species residing in different molecular entities, a critical process in nanomaterials and hybrid device engineering.
Despite their individual significance, harnessing ICT and CT simultaneously within a single molecular system has historically posed formidable challenges. Combining these two mechanisms requires exquisite molecular design to modulate both internal electron flow and intermolecular interactions. Materials must not only facilitate efficient charge transfer but also maintain structural stability and reversibility under dynamic conditions. Achieving such a balance holds the promise of creating adaptive materials capable of selective recognition and responsive signaling, yet this frontier remains largely unexplored due to synthetic and conceptual hurdles.
At the forefront of overcoming this challenge stands the class of aromatic compounds known as pyrazinacenes. These ring-structured molecules are characterized by electron deficiency, making them adept at accepting and shuttling electrons within their conjugated frameworks. This electron-poor nature equips pyrazinacenes to function as bridges between electron-donating and electron-accepting groups, providing an ideal scaffold to promote both intramolecular and intermolecular charge transfer phenomena. Their potential to mediate complex electron dynamics renders them fascinating candidates for developing hybrid ICT-CT materials.
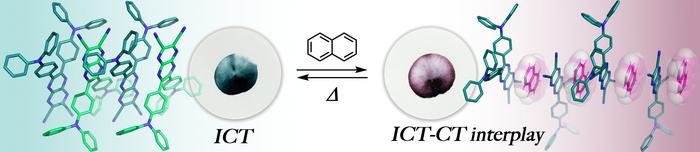
When co-crystallized with naphthalene in a strict 1:1 ratio, compound 1 exhibited an extraordinary chromatic transformation, shifting from a greenish-blue hue to an intense red-violet shade. Intriguingly, this color change was highly selective to naphthalene, as similar compounds such as octafluoronaphthalene failed to induce co-crystallization, likely due to unfavorable electronic repulsion. The selective co-crystallization was confirmed through thermogravimetric analysis and powder X-ray diffraction, which provided clear evidence of the formation of a stable hybrid crystal system reliant on specific molecular recognition.
Advanced density functional theory (DFT) calculations shed light on the underlying electronic mechanisms driving this color transition. The theoretical models revealed that the presence of naphthalene promotes an intermolecular CT event that disrupts the original ICT within compound 1. This dynamic competition between CT and ICT causes a distinctive blue shift in electronic transitions, manifested visually as the dramatic change in crystal color. Such finely tuned electron transfer interplay underscores the system’s potential as a platform for responsive molecular sensors.
Delving deeper into the molecular architecture, crystallographic analyses unveiled the fundamental role of π-hole···π interactions in stabilizing the co-crystals. In this interplay, the hydrogen atoms of naphthalene approach the electron-deficient nitrogen atoms of the pyrazinacene, facilitating non-covalent attraction without forming classic hydrogen bonds. Instead, the crystal lattice is stabilized through relatively weak Van der Waals forces, which are sufficiently dynamic to allow the system to reversibly bind and release naphthalene molecules.
This reversible binding is not only a scientific marvel but also a practical advantage. Heating the red-violet crystals to 180 °C results in the dissociation of naphthalene, restoring the original greenish-blue coloration of compound 1. This thermal reversibility underpins the material’s potential as a reusable and robust sensor capable of real-time detection and recovery, critical features for monitoring environmental contaminants such as naphthalene in aquatic ecosystems.
The significance of this work extends to environmental monitoring, where sensitive detection of trace pollutants is paramount. Naphthalene, often found in industrial effluents and combustion byproducts, is subject to increasing regulatory scrutiny due to its toxicity and persistence. The newly developed pyrazinacene-based sensor offers a straightforward visual cue for the presence of naphthalene, simplifying detection protocols without the need for complex instrumentation. This attribute represents a meaningful advancement in environmental chemistry and pollution control technologies.
“Our molecular design successfully orchestrates a delicate competition between intramolecular and intermolecular charge transfer,” explains Kazushi Nakada, the study’s first author and graduate student at SIT. “This capability empowers the sensor to selectively identify even trace levels of naphthalene in complex aqueous environments such as freshwater and seawater, providing a promising tool for environmental safety.”
Professor Akiko Hori highlights the broader implications of the research: “This study lays the groundwork for the synthesis of nonporous, adaptive crystals featuring reversible color-changing capabilities. Such materials are poised to catalyze the evolution of sensor technology and selective molecular recognition, particularly in the realm of environmental applications.” Her perspective points toward future exploration of pyrazinacene derivatives in multifunctional materials, blending responsiveness with stability and specificity.
This research represents a compelling convergence of supramolecular chemistry, crystallography, and materials science, pushing the boundaries of how molecular interactions can be tailored to produce macroscopic and controllable phenomena. The ability to induce and modulate charge transfer events in a reversible, selective manner within crystalline architectures may soon enable innovative devices that are not only sensitive and selective but also sustainable and adaptable.
As the scientific community continues to seek efficient ways to translate nanoscale interactions into practical technologies, the development of compound 1 and its unique charge transfer interplay stands as a beacon for future innovations. The integration of complex molecular engineering with accessible sensing applications enhances our toolkit for addressing pressing environmental challenges through chemistry-driven solutions.
Subject of Research:
Charge transfer mechanisms and molecular sensing using pyrazinacene derivatives.
Article Title:
Colorimetric Detection of Naphthalene Enabled by Intra-to Intermolecular Charge Transfer Interplay Induced by π-hole···π Interactions of a TPA-Attached Pyrazinacene
News Publication Date:
25 May 2025
Web References:
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202404487
References:
Nakada, K., Hori, A., Richards, G.J. (2025). Colorimetric Detection of Naphthalene Enabled by Intra-to Intermolecular Charge Transfer Interplay Induced by π-hole···π Interactions of a TPA-Attached Pyrazinacene. Chemistry – A European Journal, 31(18). DOI: 10.1002/chem.202404487
Image Credits:
Prof. Akiko Hori, Shibaura Institute of Technology, Japan
Keywords
Charge transfer, Intramolecular charge transfer, Intermolecular charge transfer, Pyrazinacene, Colorimetric sensor, Molecular recognition, π-hole interactions, Supramolecular chemistry, Crystal engineering, Environmental sensing, Naphthalene detection, Reversible color change
Tags: charge transfer phenomenacolorimetric sensorsenvironmental pollutant detectionhybrid charge transfer crystalintermolecular charge transferintramolecular charge transfermaterial science advancementsnaphthalene sensing technologyoptical and electronic propertiesorganic electronics applicationspyrazinacene derivativesreversible color-changing materials