Scientists at Stanford University School of Medicine and Sanford Burnham Prebys have demonstrated a new way to accelerate recovery from peripheral nerve injury by targeting an enzyme that was thought to be responsible for muscle wasting with aging.
Credit: Blau lab, Stanford University School of Medicine
Scientists at Stanford University School of Medicine and Sanford Burnham Prebys have demonstrated a new way to accelerate recovery from peripheral nerve injury by targeting an enzyme that was thought to be responsible for muscle wasting with aging.
Damage to the peripheral nervous system (the nerves that form the communications network between the brain, spinal cord and body) is debilitating; the effectiveness of physiotherapy as treatment is limited. Whether from trauma, disease or aging, nerve function declines and/or is lost, resulting in diminished strength and even paralysis.
In the new study, published October 11, 2023 online in Science Translational Medicine and co-authored by Helen M. Blau, Ph.D., the Donald E. and Delia B. Baxter Foundation Professor at Stanford University School of Medicine, and Yu Xin Wang, Ph.D., assistant professor in the Development, Aging and Regeneration Program at Sanford Burnham Prebys, the research team found that an enzyme associated with aging is triggered by the loss of innervation.
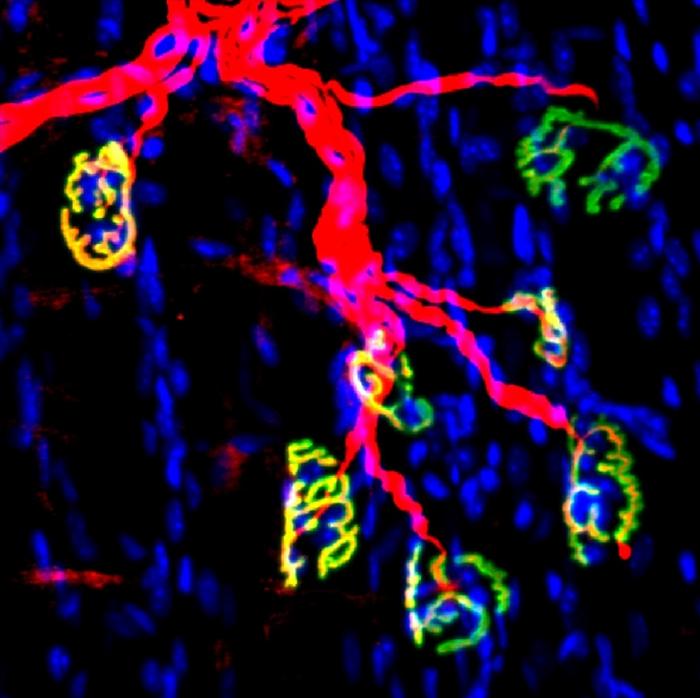
Inhibiting this enzyme after nerve injury in a mouse model with a small molecule inhibitor promoted regeneration of motor nerve and formation of neuromuscular synapses that resulted in accelerated recovery of strength.
“Our data suggests that inhibiting the function of this particular enzyme, called 15-hydroxyprostaglandin dehydrogenase or 15-PGDH, with a small molecule boosted a naturally occurring compound (prostaglandin E2 or PGE2) in muscle tissues that helps restore nerve connectivity, function and strength,” said Wang.
When skeletal muscles lose nerve function, a phenomenon called denervation, they atrophy and weaken. Denervation can result from physical trauma, such as damage to nerves connecting muscles to the spinal cord, or from heritable neuromuscular wasting diseases, such as amyotrophic lateral sclerosis. Advanced age can also lead to severe muscle wasting called sarcopenia.
Muscle denervation and its consequences affect up to an estimated 5% of the U.S. population (approximately 17 million persons) and result in annual estimated national health care costs of $380 billion.
Wang was a part of Blau’s team at Stanford University, who had shown in previous research that PGE2 in muscle tissues is required for muscle stem cells to proliferate and for muscles to effectively regenerate and repair after injury.
The researchers have also found that 15-PGDH degrades PGE2 and that the enzyme accumulates with age. They have dubbed 15-PGDH a gerozyme — an enzyme that determines muscle wasting and which increases with aging.
“We wondered why this enzyme turns on with age if it has such a negative impact on muscle mass and strength,” said Wang.
The new work involved studying young mice using surgical methods to model injuries to the sciatic nerve. Levels of 15-PGDH rose in the denervated muscles, but pharmacological inhibition of 15-PGDH promoted subsequent motor axon growth, neuromuscular connectivity and faster recovery.
In studies of human tissues, the researchers detected aggregates of 15-PGDH in biopsies from a diverse range of human neuromuscular diseases, suggesting that inhibiting this enzyme could be beneficial.
“Restoring neuromuscular connectivity is a critical step in treating these debilitating disorders. This new approach is attractive because the treatment signals the nerve to grow back,” said Wang. “That’s why it has such a profound effect on the muscle and strength.”
Additional authors on the study include Mohsen A. Bakooshli, Elena Monti, Shiqi Su, Peggy Kraft, Minas Nalbandian, Ludmila Alexandrova, Joshua R. Wheeler and Hannes Vogel, all at Stanford University.
This study was supported by the National Institutes of Health Shared Instrumentation Grant (S100D026962); the Canadian Institutes of Health (MFE-152457); Stanford Translational Research and Applied Medicine Pilot grant; National Institutes of Health (K99NS120278, R00NS120278, R01-AG020961, R01-AG069858, R01-G009674), Stanford Dean of Research—SUMS Seed Grant Program; Donald E. and Delia B. Baxter Foundation; the Li Ka Shing Foundation; Milky Way Research Foundation (MWRF-21664), California Institute for Regenerative Medicine (DISC2-10604).
Disclosure: Authors Bakooshli, Wang and Blau are named inventors on patent application “A method to restore neuromuscular junction morphology,” assigned to Stanford University.
The study’s DOI is 10.1126/scitranslmed.adg1485.
Journal
Science Translational Medicine
DOI
10.1126/scitranslmed.adg1485
Article Title
Regeneration of Neuromuscular Synapses after Acute and Chronic Denervation by Inhibiting the Gerozyme 15-Prostaglandin Dehydrogenase
Article Publication Date
11-Oct-2023




