Credit: ©ICHINOHE Takeshi
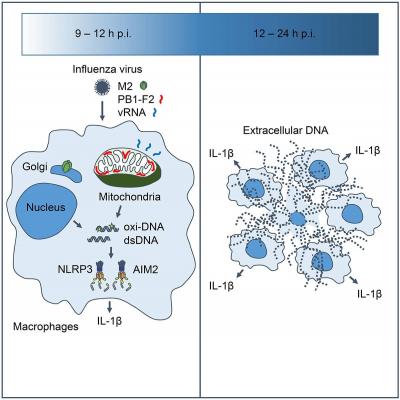
Both influenza virus M2, a proton-selective ion channel essential for efficient viral replication, and PB1-F2 protein, which localizes to the mitochondria and attenuates host antiviral immunity, are involved in the inflammatory response by stimulating NLRP3 inflammasome-dependent IL-1β secretion. However, the precise mechanisms by which these viral proteins activate the NLRP3 inflammasome remain unclear.
In this study, a research group at The Institute of Medical Science, The University of Tokyo (IMSUT) observed nucleus- and mitochondria-derived double-stranded DNA (dsDNA) in extracellular web-like structures in the cytoplasm and extracellular space around influenza virus-infected macrophages.
According to lead scientist Takeshi Ichinohe, Associate Professor, Division of Viral Infection of IMSUT, Oxidized DNA was included in these DNAs, and the research group found that the ion channel activity of the M2 protein and mitochondrial localization of the PB1-F2 protein is required for oxidized DNA release. While the oxidized DNA enhanced influenza virus-induced IL-1β secretion, inhibition of mitochondrial reactive oxygen species decreased the virus-induced IL-1β secretion. In addition, they showed that influenza virus stimulates IL-1β secretion from macrophages in a manner that is dependent on AIM2, a cytosolic DNA sensor. These results provide a missing link between influenza viral proteins and NLRP3 inflammaseome activation, and reveal the importance of influenza virus-induced oxidized DNA in inflammasomes activation.
###
Media Contact
ICHINOHE Takeshi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




