Cardiovascular diseases (CVDs) or heart diseases are still the leading cause of death globally. Fortunately, doctors are now equipped with more advanced and sophisticated tools that help them diagnose CVDs. A prominent example is intravascular ultrasound (IVUS), which enables cardiologists to obtain images of the inside of blood vessels using a thin ultrasound probe. These images can then be used to assess problems such as the thickening of arteries caused by fat or plaque buildup.
Credit: Rauschendorfer et al., doi 10.1117/1.JBO.28.4.04600.
Cardiovascular diseases (CVDs) or heart diseases are still the leading cause of death globally. Fortunately, doctors are now equipped with more advanced and sophisticated tools that help them diagnose CVDs. A prominent example is intravascular ultrasound (IVUS), which enables cardiologists to obtain images of the inside of blood vessels using a thin ultrasound probe. These images can then be used to assess problems such as the thickening of arteries caused by fat or plaque buildup.
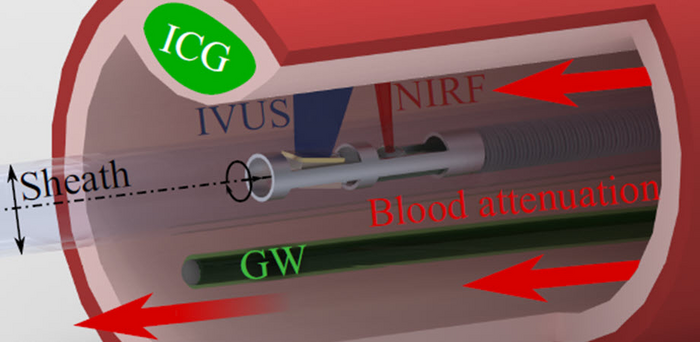
While IVUS is undoubtedly a powerful technique, it fails to capture key information about the pathology of the observed vessels. To address this problem, near-infrared fluorescence (NIRF) imaging is used in conjunction with IVUS for a more thorough examination of the blood vessels. NIRF makes use of fluorescent agents that can outline biological processes inside the body. These agents are injected into the bloodstream, where they bind to specific pathology-related compounds on vessel walls, such as proteins or nucleic acids. The fluorescence signals that are generated are combined with the IVUS images to provide accurate information. However, during NIRF-IVUS measurements, the distance between the NIRF detector and the blood vessel wall keeps changing. This presents a new challenge because blood attenuates the intensity of the fluorescence signals, and the “amount” of blood between the NIRF detector and the vessel wall varies constantly.
Thus, a team of researchers led by Professor Vasilis Ntziachristos from the Technical University of Munich, Germany, came up an innovative solution to this problem. In a new study published in Journal of Biomedical Optics (JBO), the team reported a new technique to measure the fluorescence attenuation of blood using a “guidewire” that moves the NIRF-IVUS probe. “We provide an adaptive correction scheme tailored to each patient and each imaging frame collected during the imaging procedure,” says Ntziachristos.
The idea behind this new method is based on the fact that the guidewire is always visible to the NIRF probe. Coating the guidewire with a known concentration of fluorescent particles ensures that the signal on the guidewire will provide an indirect measure of the blood attenuation in the current image. The distance between the NIRF probe and the guidewire is determined via IVUS, and so is the distance between the NIRF probe and the blood vessel wall. A correction factor for the fluorescence signal measured at the blood vessel wall can be calculated after a simple calibration procedure.
The researchers used a small NIRF-IVUS system reported in their previous study to test their technique in a clinical model. They also performed experiments on capillary phantoms, which simulate the properties of small blood vessels. They recorded a 4.5-fold improvement over uncorrected NIRF signal and <11 percent errors for target signals, which looks quite promising! Moreover, the correction method maintained a mean accuracy of 70 percent in tissue experiments. These values are also in stark contrast to the accuracies obtained by other correction methods, which use average attenuation factors rather than calculating them for each frame and for the precise probe-to-vessel distances measured via IVUS.
The team suggests that it should be relatively easy to directly incorporate their technique into clinical practice, since no major modifications to existing equipment are required. The guidewire can be used as a reference standard for other intravascular fluorescence imaging modalities, as well as other optical methods beyond fluorescence, if appropriate coatings are used.
Professor of Medical Physics at the University of Wisconsin – Madison and JBO Editor-in-Chief Brian Pogue remarks, “This new method for correcting intravascular NIRF signals is simple and accurate and could pave the way for in vivo studies and eventual clinical translation.”
Read the Gold Open Access article by Philipp Rauschendorfer et al., “Accounting for blood attenuation in intravascular near-infrared fluorescence-ultrasound imaging using a fluorophore-coated guidewire,” J. Biomed. Opt. 28(4), 046001 (2023), doi 10.1117/1.JBO.28.4.046001.
Journal
Journal of Biomedical Optics
DOI
10.1117/1.JBO.28.4.046001
Article Title
Accounting for blood attenuation in intravascular near-infrared fluorescence-ultrasound imaging using a fluorophore-coated guidewire
Article Publication Date
5-Apr-2023




