Since the outbreak of COVID-19, the importance wearing masks and disinfection of items has become paramount. As a result, there is now a greater need for effective, potent, and simple-to-apply disinfectants. Foam-type disinfectants are a leading candidate in this regard since they do not drip, keep the disinfected area visible, and are less likely to reach the user’s eyes.
Credit: Kenichi Sakai from Tokyo University of Science
Since the outbreak of COVID-19, the importance wearing masks and disinfection of items has become paramount. As a result, there is now a greater need for effective, potent, and simple-to-apply disinfectants. Foam-type disinfectants are a leading candidate in this regard since they do not drip, keep the disinfected area visible, and are less likely to reach the user’s eyes.
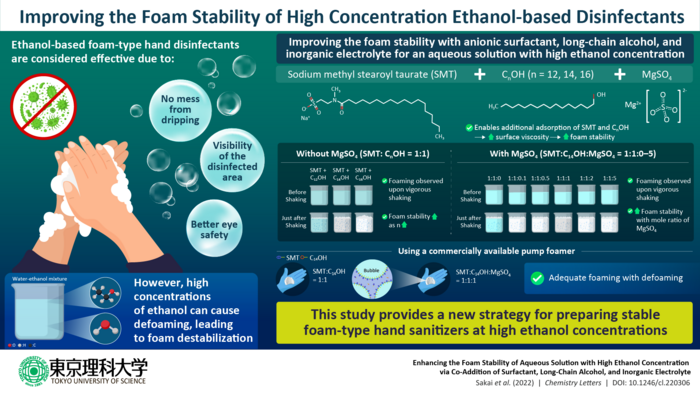
However, foam-type disinfectants are not without issues. While the foam is usually stabilized with the adsorption of a surfactant at the air/liquid interface, adding high concentration of ethanol, an antiseptic, to foams in aqueous solutions causes defoaming resulting from destabilization of the foam.
To improve the stability of foam disinfectants at high ethanol concentrations, a group of researchers from Tokyo University of Science (TUS), Japan, in collaboration with the Life Science Products Division, NOF Corporation, have now come up with a new proposal. This study, led by Associate Professor Kenichi Sakai of TUS, was made available online on August 04, 2022 and published in Chemistry Letters.
In their study, the team added an anionic (negatively charged) surfactant, long-chain alcohols, and an inorganic electrolyte to an aqueous solution containing 60% ethanol by volume. They used sodium methyl stearoyl taurate (SMT) as the surfactant, CnOH (where n = 12, 14, 16) as the alcohols, and magnesium sulfate (MgSO4) as the electrolyte.
The inorganic electrolyte provided two main advantages: firstly, it enabled effective screening of the electrostatic repulsion between the SMT headgroup adsorbed at the air-liquid interface. Secondly, it promoted interactions between Mg2+ ions and the headgroups. These, in turn, facilitated the additional adsorption of SMT and CnOH, increasing the surface viscosity and foam stability.
“We have been working on this research project before the novel coronavirus infection became a social problem. We believe that the social impact of this research will only increase as the social need for disinfectants and health safety go up,” says Dr. Sakai, explaining his motivation behind the study.
The team observed that, in the absence of the MgSO4, foaming occurred upon shaking for CnOH (n = 12, 14, 16) with the foam stability increasing with increasing n. Additionally, the combination of SMT and CnOH resulted in a decrease in surface tension and an increase in surface viscosity, which increased foam stability.
When MgSO4 was added, foaming happened upon vigorous shaking. The foam stability increased with increase in the mole ratio of MgSO4, which decreased the surface tension while increasing the surface viscosity.
Finally, the team used a non-pressurized commercial pump to test the foam formation of the solution. They found that the SMT and C14OH mixture produced adequate foaming both with and without MgSO4. Further, defoaming occurred after 30 seconds for both cases, an appropriate time scale for the dissipation of the foam after application.
“The COVID-19 pandemic has seriously affected human lives and social activities on a global scale. As a result, the importance of proper sanitation has been recognized worldwide. We believe that the results of our research will contribute to the sustainable development goal (SDG3) of ensuring good health and well-being among people of all ages,” says Dr. Sakai.
Indeed, the team’s samples could help formulate foam-type hand sanitizers you may be using soon!
***
Reference
DOI: https://doi.org/10.1246/cl.220306
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Associate Professor Kenichi Sakai from Tokyo University of Science
Dr. Kenichi Sakai is an Associate Professor at the Tokyo University of Science, Japan, where he is a member of the Faculty of Science and Technology in the Department of Pure and Applied Chemistry. His research interests lie in the field of physical chemistry, particularly in colloid and interface chemistry. He has over 160 publications to his name and has received several awards, including the Young Scientist Award from the Division of Colloid and Surface Chemistry, The Chemical Society of Japan in 2015, and the Coating Societies International Medallion in 2007.
Funding information
This study was supported by the JST A-STEP (Adaptable and Seamless Technology transfer Program through target-driven R&D) Grant Number JPMJTM20CF. SMT was kindly provided by Nikko Chemicals Co., Ltd.
Journal
Chemistry Letters
DOI
10.1246/cl.220306
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Enhancing the Foam Stability of Aqueous Solution with High Ethanol Concentration via Co-Addition of Surfactant, Long-Chain Alcohol, and Inorganic Electrolyte
Article Publication Date
4-Aug-2022
COI Statement
The authors have no conflicts to declare




