Researchers at Karlsruhe Institute of Technology (KIT) have made significant advances in sodium-ion battery (SIB) technology by improving cycling performance of the NaNiO2 cathode. They successfully synthesized, for the first time, the cathode active material NaNi0.9Ti0.1O2, which delivers a specific capacity of 190 mAh/g, thus positioning it as a potential candidate for application in high-energy-density SIBs. This innovative approach not only improves battery stability but also propels us toward advanced energy-storage solutions beyond.
Credit: Siyu An and Torsten Brezesinski from Battery and Electrochemistry Laboratory (BELLA), Institute of Nanotechnology, Karlsruhe Institute of Technology (KIT)
Researchers at Karlsruhe Institute of Technology (KIT) have made significant advances in sodium-ion battery (SIB) technology by improving cycling performance of the NaNiO2 cathode. They successfully synthesized, for the first time, the cathode active material NaNi0.9Ti0.1O2, which delivers a specific capacity of 190 mAh/g, thus positioning it as a potential candidate for application in high-energy-density SIBs. This innovative approach not only improves battery stability but also propels us toward advanced energy-storage solutions beyond.
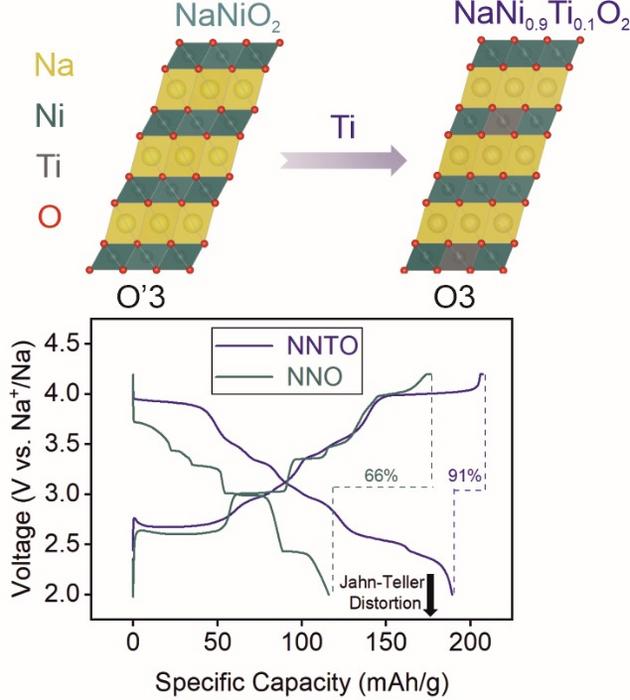
With its high theoretical specific capacity, NaNiO2 (NNO) shows great potential as an O3-type sodium-ion battery material for beyond lithium energy-storage applications. However, the exchanges of the large Na+ ions can cause severe interlayer sliding and volume changes, reducing cycling performance. Additionally, the Jahn-Teller distortion induced by Ni3+, an uneven arrangement of electrons around the ion’s orbitals, adversely impacts long-term cyclability. Addressing these issues can significantly enhance the practical application of NNO in the near future.
The Solution: A research team from Karlsruhe Institute of Technology (KIT) successfully introduced 10 mol% Ti4+ into the Ni site of NNO. This helps to maintain a larger interslab distance in the Na-deficient phases and to mitigate Jahn-Teller activity by reducing the average oxidation state of Ni.
The Future:Although NaNi0.9Ti0.1O2 (NNTO) shows significant improvements in cycling performance over NNO, it still faces issues of large volume variations during battery operation and irreversible lattice oxygen loss at high potentials. These problems lead to structural instability and capacity decay. To address the electro-chemo-mechanical degradation/failure, dopants can be introduced into the Na and/or transition-metal sites of NNTO.
The Impact:By combining physical and electrochemical characterization techniques, insights into the potential reasons behind the capacity fading of NNTO are gained, offering new avenues for tailoring this promising cathode active material. The findings are expected to have broad implications for the sodium-ion battery by providing a novel material for high-energy-density, electrochemical energy-storage applications.
This work has been recently published in the online edition of Materials Futures, a prominent international journal in the field of interdisciplinary materials science research.
Reference
Siyu An, Leonhard Karger, Sören L. Dreyer, Yang Hu, Eduardo Barbosa, Ruizhuo Zhang, Jing Lin, Maximilian Fichtner, Aleksandr Kondrakov, Jürgen Janek and Torsten Brezesinski, “Improving Cycling Performance of the NaNiO2 Cathode in Sodium-Ion Batteries by Titanium Substitution” 2024, DOI: 10.1088/2752-5724/ad5faa.
Journal
Materials Futures
DOI
10.1088/2752-5724/ad5faa
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Improving Cycling Performance of the NaNiO2 Cathode in Sodium-Ion Batteries by Titanium Substitution
Article Publication Date
5-Jul-2024