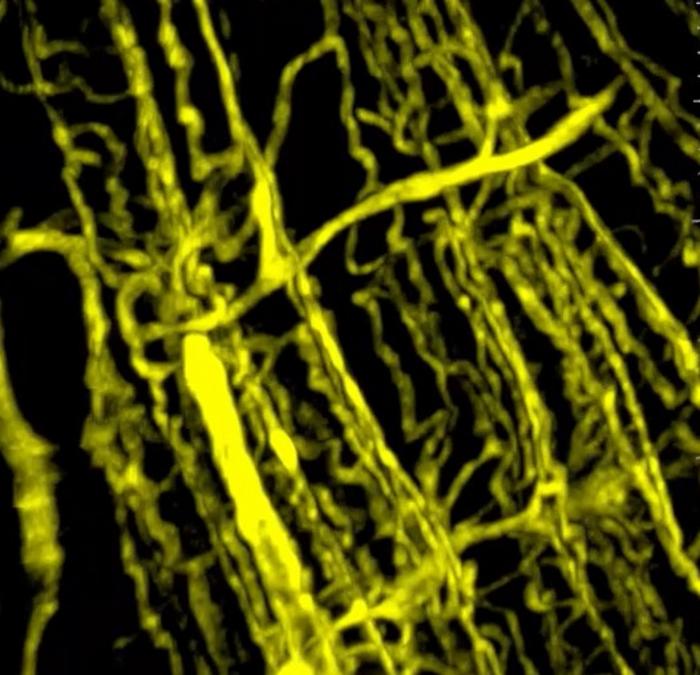
A groundbreaking study conducted by researchers at the University of Illinois Chicago has unveiled a critical link between muscle blood vessel dysfunction and the debilitating muscle weakness and weight loss experienced by cancer patients. This phenomenon, known as cancer cachexia, severely impairs quality of life and survival outcomes for many individuals battling this disease. The team’s findings shed light on the pivotal role of vascular health in maintaining muscle integrity and suggest novel therapeutic avenues for reversing cachexia, a condition that currently lacks effective treatment.
Cancer cachexia affects up to 80% of patients, leading to severe muscle wasting, profound fatigue, and dramatic weight loss. Despite its prevalence and impact, the mechanisms driving cachexia have remained elusive, with existing interventions focusing primarily on nutritional support and physical activity showing limited success. This new research, led by Dr. Jalees Rehman, head of the Department of Biochemistry and Molecular Genetics at UIC, challenges prior assumptions by highlighting the molecular interplay within muscle blood vessels as a key driver of muscle degradation in cancer.
.adsslot_65Hzc08wgx{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_65Hzc08wgx{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_65Hzc08wgx{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Using advanced three-dimensional microscopy techniques, the researchers compared blood vessel networks in muscle tissues from animal models of pancreatic cancer as well as samples from human cancer patients. They observed a marked reduction in vascular density within the cachexic muscles compared to healthy controls. This compromised vascular network correlated with smaller muscle size and diminished contractile strength, underscoring the vital importance of vascular integrity in muscle health during cancer progression.
Furthermore, gene expression profiling of endothelial cells extracted from these muscles demonstrated signs of premature cellular aging, increased permeability, and heightened susceptibility to apoptosis. These pathological changes impair the ability of blood vessels to support muscle maintenance and regeneration. Notably, activin A levels were elevated in these dysfunctional endothelial cells, directly linking tumor-secreted factors to vascular deterioration.
Significantly, the research team discovered that reactivating PGC1α within the endothelial cells could restore vascular function and reverse muscle wasting. This finding indicates that the vascular abnormalities driving cachexia are not irreversible and opens new therapeutic possibilities. By targeting the activin A–PGC1α axis, it may be possible to develop treatments that halt or even reverse muscle loss, thereby improving patient strength and survival.
This research moves beyond the conventional focus on inflammation-driven muscle damage and introduces blood vessel health as a critical factor in cancer cachexia. It suggests that therapies aimed at preserving or restoring vascular function could complement nutritional and exercise strategies, potentially offering a more comprehensive approach to managing this complex syndrome.
The implications extend beyond pancreatic cancer, as similar blood vessel dysfunction and muscle wasting were observed in models of colon, lung, and skin melanoma cancers. This broad relevance highlights the commonality of vascular mechanisms in cancer-associated cachexia across tumor types.
While the precise molecular cascades initiated by activin A within endothelial cells are still being mapped, the central role of PGC1α as a metabolic regulator places this protein at the forefront of future research. Modulating PGC1α expression or function could prove essential not just for cancer cachexia but also for other muscle-wasting disorders linked to vascular impairment.
Overall, this study presents a compelling case for reconsidering muscle blood vessels as active participants in cancer-related muscle loss rather than passive conduits. Their dysfunction appears to precipitate and perpetuate cachexia, making them attractive targets for intervention. The research team is now actively exploring pharmacological agents and gene therapies aimed at modulating the activin A–PGC1α signaling axis to translate these findings into clinical applications.
Dr. Rehman emphasizes that addressing the vascular component of cachexia could dramatically improve the lives of cancer patients, enabling them to maintain muscle strength, independence, and resilience during treatment and recovery. As this new paradigm gains traction, it holds promise for tackling one of oncology’s most challenging complications with innovative, mechanism-based therapies.
In conclusion, the University of Illinois Chicago researchers have illuminated a previously underappreciated pathway underpinning cancer cachexia: endothelial dysfunction driven by the activin A–PGC1α axis. By restoring vascular health within skeletal muscle, there is potential not only to halt muscle wasting but also to improve overall patient outcomes markedly. This discovery paves the way for future clinical trials and heralds a new chapter in the fight against muscle loss in cancer.
Subject of Research: Skeletal muscle endothelial dysfunction in cancer cachexia
Article Title: Skeletal muscle endothelial dysfunction through the activin A–PGC1α axis drives progression of cancer cachexia
News Publication Date: 26-May-2025
Web References: http://dx.doi.org/10.1038/s43018-025-00975-6
Image Credits: (Image: Nature Cancer, reprinted under Creative Commons license)
Keywords: Health and medicine, Cancer, Muscle diseases
Tags: activin A in cancercancer cachexia mechanismscancer patient survival outcomesendothelial cells and muscle degradationimpaired blood vessel functionmuscle wasting in cancernovel treatments for muscle weaknessQuality of Life in Cancer Patientstherapeutic avenues for cachexiaUniversity of Illinois Chicago researchvascular health and muscle integrityweight loss and fatigue in cancer