New York, NY [March 21, 2024]—Using novel genetic and genomic tools, researchers at the Icahn School of Medicine at Mount Sinai have shed light on the role of immune cells called macrophages in lipid-rich tissues like the brain, advancing our understanding of Alzheimer’s and other diseases. The study, published in the March 6 online issue of Nature Communications [DOI: 0.1038/s41467-024-46315-7], represents a step forward in understanding immune cell regulation and its impact on disease progression.
Credit: Lab of Alison M. Goate, DPhil.
New York, NY [March 21, 2024]—Using novel genetic and genomic tools, researchers at the Icahn School of Medicine at Mount Sinai have shed light on the role of immune cells called macrophages in lipid-rich tissues like the brain, advancing our understanding of Alzheimer’s and other diseases. The study, published in the March 6 online issue of Nature Communications [DOI: 0.1038/s41467-024-46315-7], represents a step forward in understanding immune cell regulation and its impact on disease progression.
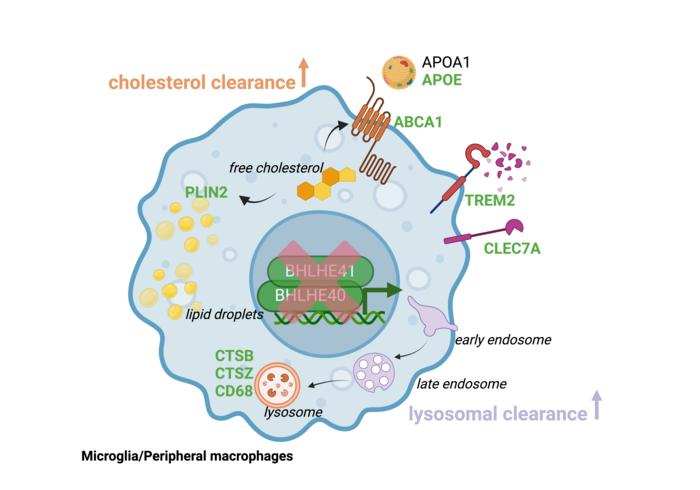
The researchers initially studied genes controlling macrophages, also referred to as microglia when they are in the brain, particularly in response to damage of fatty tissues like the brain. Disease-associated microglia/macrophages are thought to be protective, as they participate in removing lipid-rich waste derived from tissue damage. Therefore, researchers wanted to find factors that promote the “garbage removal” activity of these cells.
They identified two influential genes, BHLHE40 and BHLHE41, and used advanced gene editing technology (CRISPR-Cas9) to deactivate them in lab-grown cells. These cells were then transformed into microglia. The resulting microglia lacking BHLHE40 and BHLHE41 resembled disease-associated microglia observed in Alzheimer’s disease, showing improved ability to clear cholesterol-rich waste. Confirmation came from experiments on cultured human peripheral macrophages and microglia from mice lacking these genes.
“Through our analysis of single-cell datasets from multiple organs, we’ve uncovered pivotal regulators of immune cell function essential for tissue health,” says senior study author Alison M. Goate, DPhil, the Jean C. and James W. Crystal Professor and Chair of Genetics and Genomic Sciences at Icahn Mount Sinai. “Our use of advanced models has further validated the critical role played by transcription factors BHLHE40 and BHLHE41, proteins that regulate gene expression by binding to specific DNA sequences, in controlling immune cell responses, presenting potential targets for therapeutic intervention.”
Next, the researchers will investigate whether microglia without BHLHE40 and BHLHE41 can help clear away harmful amyloid proteins. In one experiment, the investigators will grow brain cells such as neurons and astrocytes that have harmful Alzheimer’s mutations in a dish and test whether immune cells without BHLHE40 and 41 influence beta-amyloid levels, neurodegeneration, and cytokine response (neuroinflammation). In another, they will inject the immune cells with and without BHLHE40 and 41 into a mouse model of Alzheimer’s to see how they affect the development of Alzheimer’s-like plaques.
“We want to see how these cells, particularly those without the two genes, impact Alzheimer’s-related phenotypes in both dish and mouse models. We predict that in mice, microglia without BHLHE40 and 41 will clear away beta-amyloid plaques more effectively than control microglia that have normal levels of BHLHE40 and 41. Also, we’re exploring how lack of these genes in the brain immune cells affect other types of cells in the brain such as neurons and astrocytes,” says Dr. Goate.
The paper is titled “BHLHE40/41 regulate microglia and peripheral macrophage responses associated with Alzheimer’s disease and other disorders of lipid-rich tissues.”
The remaining authors of the paper, all with Icahn Mount Sinai except where indicated, are: Anna Podlesny-Drabiniok, PhD; Gloriia Novikova, PhD; Yiyuan Liu, PhD; Josefine Dunst, PhD (Anocca in Sweden); Rose Temizer, PhD candidate (National Institute of Mental Health); Samuele Marro, PhD; Taras Kreslavskiy, PhD (Karolinska Institute); and Edoardo Marcora, PhD.
Please see DOI: 0.1038/s41467-024-46315-7 to view more details on the paper and competing interests.
The study was made possible by funding from various sources, including NIH grants (RF1AG054011, U01AG058635, R56AG081417, U01AG066757, NHLBI R01HL153712, S10OD026880 and S10OD030463) and support from The JPB Foundation and BrightFocus Foundation (A2021014F), the New York State Department of Health stem cell biology fellowship (NYSTEM- C32561GG), America Heart Association (20SFRN35210252), and Graduate Women in Science Fellowship.
-####-
About the Icahn School of Medicine at Mount Sinai
The Icahn School of Medicine at Mount Sinai is internationally renowned for its outstanding research, educational, and clinical care programs. It is the sole academic partner for the eight- member hospitals* of the Mount Sinai Health System, one of the largest academic health systems in the United States, providing care to a large and diverse patient population.
Ranked 13th nationwide in National Institutes of Health (NIH) funding and among the 99th percentile in research dollars per investigator according to the Association of American Medical Colleges, Icahn Mount Sinai has a talented, productive, and successful faculty. More than 3,000 full-time scientists, educators, and clinicians work within and across 44 academic departments and 36 multidisciplinary institutes, a structure that facilitates tremendous collaboration and synergy. Our emphasis on translational research and therapeutics is evident in such diverse areas as genomics/big data, virology, neuroscience, cardiology, geriatrics, as well as gastrointestinal and liver diseases.
Icahn Mount Sinai offers highly competitive MD, PhD, and Master’s degree programs, with current enrollment of approximately 1,300 students. It has the largest graduate medical education program in the country, with more than 2,000 clinical residents and fellows training throughout the Health System. In addition, more than 550 postdoctoral research fellows are in training within the Health System.
A culture of innovation and discovery permeates every Icahn Mount Sinai program. Mount Sinai’s technology transfer office, one of the largest in the country, partners with faculty and trainees to pursue optimal commercialization of intellectual property to ensure that Mount Sinai discoveries and innovations translate into healthcare products and services that benefit the public.
Icahn Mount Sinai’s commitment to breakthrough science and clinical care is enhanced by academic affiliations that supplement and complement the School’s programs.
Through the Mount Sinai Innovation Partners (MSIP), the Health System facilitates the real-world application and commercialization of medical breakthroughs made at Mount Sinai. Additionally, MSIP develops research partnerships with industry leaders such as Merck & Co., AstraZeneca, Novo Nordisk, and others.
The Icahn School of Medicine at Mount Sinai is located in New York City on the border between the Upper East Side and East Harlem, and classroom teaching takes place on a campus facing Central Park. Icahn Mount Sinai’s location offers many opportunities to interact with and care for diverse communities. Learning extends well beyond the borders of our physical campus, to the eight hospitals of the Mount Sinai Health System, our academic affiliates, and globally.
——————————————————-
* Mount Sinai Health System member hospitals: The Mount Sinai Hospital; Mount Sinai Beth Israel; Mount Sinai Brooklyn; Mount Sinai Morningside; Mount Sinai Queens; Mount Sinai South Nassau; Mount Sinai West; and New York Eye and Ear Infirmary of Mount Sinai.
Journal
Nature Communications
Method of Research
Experimental study
Subject of Research
Cells
Article Title
BHLHE40/41 regulate microglia and peripheral macrophage responses associated with Alzheimer’s disease and other disorders of lipid-rich tissues
Article Publication Date
6-Mar-2024




