The enzyme TBK1 is an important component of the innate immune system that plays a critical role in the defense against viruses. Upon mutation-induced loss of TBK1 function, patients show an increased susceptibility to viral infections. Strikingly, if TBK1 is not expressed at all, this clinical effect is not seen. The mechanism behind this supposed discrepancy has now been elucidated by researchers led by Prof. Martin Schlee from the University Hospital Bonn and the Cluster of Excellence ImmunoSensation2 at the University of Bonn. The study was published in the journal Frontiers in Immunology.
Credit: Frontiers in Immunology/DOI: 10.3389/fimmu.2023.1073608
The enzyme TBK1 is an important component of the innate immune system that plays a critical role in the defense against viruses. Upon mutation-induced loss of TBK1 function, patients show an increased susceptibility to viral infections. Strikingly, if TBK1 is not expressed at all, this clinical effect is not seen. The mechanism behind this supposed discrepancy has now been elucidated by researchers led by Prof. Martin Schlee from the University Hospital Bonn and the Cluster of Excellence ImmunoSensation2 at the University of Bonn. The study was published in the journal Frontiers in Immunology.
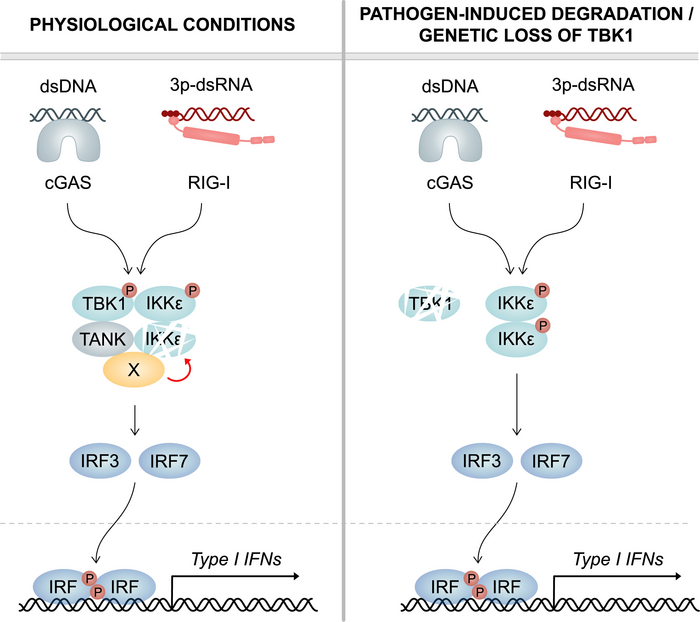
In the human body, viral particles are recognized by so called pattern recognition receptors (PRRs) situated within the cell or on the cell surface. Upon activation, a signaling cascade is initialized which ultimately results in the production and release of signaling molecules such as interferons and cytokines. These messenger molecules alert neighboring immune cells and point out the viral infection, inducing an immune reaction.
Part of this signaling cascade is the TANK Binding Kinase 1 (TBK1). If viral particles are detected by PRRs, TBK1 is activated. TBK1 in turn activates two transcription factors which travel into the nucleus where they induce the transcription of interferon and cytokine genes.
Susceptibility to viral infections
Point-mutations in the TBK1 gene may induce a loss of function of TBK1. In humans, this manifests itself in clinical susceptibility to viral infections. Strikingly, this effect is not to be observed if TBK1 is not expressed and entirely lacking in the cell. “Surprisingly, a complete absence of TBK1 expression in humans is not associated with a reduced antiviral response,” says Prof. Martin Schlee of the Institute of Clinical Chemistry and Clinical Pharmacology at the University Hospital Bonn. Until now, it was unclear why a complete loss of TBK1 expression is better tolerated in terms of immunocompetence than a mutation of TBK1 affecting the kinase function.
The Bonn researchers have now been able to provide an explanation for these previously unexplained observations. “A second enzyme that is very similar to TBK1 plays an important role in this: the IkB kinase epsilon, or IKKepsilon for short,” explains Dr. Julia Wegner, first author of the study. Just like TBK1, IKKepsilon acts downstream of PRRs and controls the expression of interferons. The two proteins are also very similar in structure, with more than 60 percent sequence homology. A novel finding is that TBK1 has a direct effect on IKKepsilon. “In myeloid cells, we could show that TBK1 regulates the expression of the related kinase IKKepsilon,” adds Dr. Wegner.
No half measures
TBK1 reduces the stability of IKKepsilon. This process is independent of the protein’s enzymatic function. “Accordingly, TBK1 that is nonfunctional due to point mutation is still able to destabilize IKKepsilon,” explains Prof. Gunther Hartmann, director of the Institute of Clinical Chemistry and Clinical Pharmacology and spokesperson of the ImmunoSensation2 Cluster of Excellence. “This leads to a continuous degradation of the kinase IKKepsilon in human immune cells.”
Therefore, loss of TBK1 expression leads to an increased abundance of IKKepsilon. This mechanism ensures that an antiviral immune response can occur despite the absence of TBK1. Loss of function of TBK1 induced by point mutations, on the other hand, does not prevent destabilization and degradation of IKKepsilon, so that ultimately both factors are not available for viral defense. Increased susceptibility to viral infections is the result.
Weapons of a virus
In a healthy organism, increased amounts of IKKepsilon can thus compensate for the loss of TBK1. This becomes particularly important when viruses specifically seek to eliminate the body’s own defenses. Herpes simplex virus 1 (HSV-1), human immunodeficiency virus (HIV) but also severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are able to specifically induce TBK1 degradation. Also, several bacterial species are capable of causing the degradation of TBK1. “Our data clearly show that human immune cells have an important backup mechanism,” explains Dr. Wegner. “They are able to maintain an effective antiviral response even when pathogen-induced degradation of TBK1 occurs. Furthermore, the mechanism also takes effect in the case of genetic loss of TBK1.”
Publication: Wegner Julia, Hunkler Charlotte, Ciupka Katrin, Hartmann Gunther, Schlee Martin (2023); Increased IKKepsilon protein stability ensures efficient type I interferon responses in conditions of TBK1 deficiency; Frontiers in Immunology , Vol. 14; DOI: 10.3389/fimmu.2023.1073608
Contact:
Dr. David Fußhöller
Cluster of Excellence ImmunoSensation2
University of Bonn
Tel. +49 228 287 512 83
E-mail: [email protected]
Journal
Frontiers in Immunology
DOI
10.3389/fimmu.2023.1073608
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Increased IKKepsilon protein stability ensures efficient type I interferon responses in conditions of TBK1 deficiency
Article Publication Date
3-Mar-2023




