Credit: Scientific Reports; Perelman School of Medicine, University of Pennsylvania
Symptoms and efficacy of medications – and indeed, many aspects of the human body itself — vary by time of day. Physicians tell patients to take their statins at bedtime because the related liver enzymes are more active during sleep. Studies have also identified that most heart attacks occur in the early morning as the body jolts awake. Often, studies on the 24-hour rhythms of human physiology focus on many patients, but few parameters.
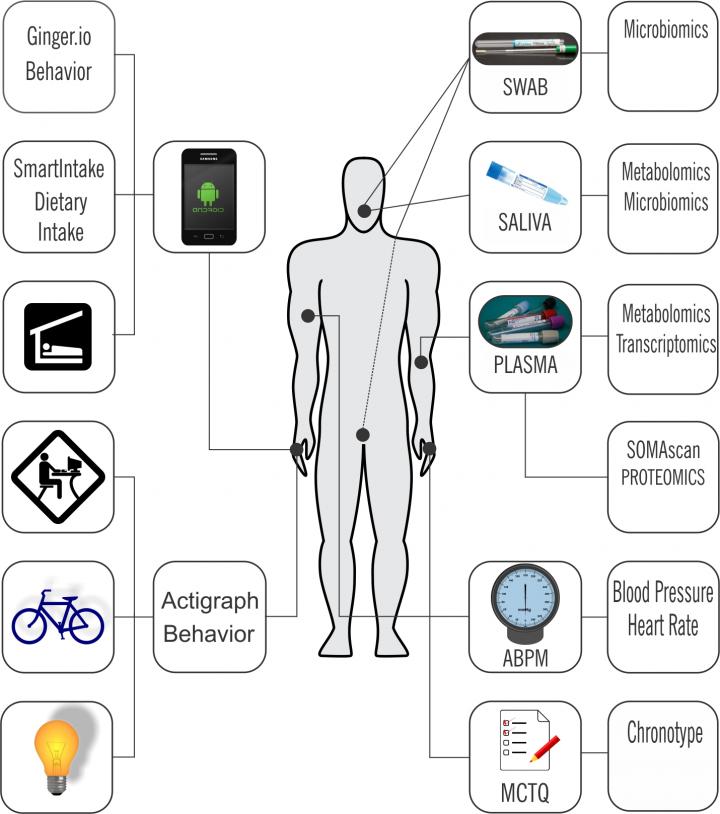
A study reported in Scientific Reports set this approach on its head. Researchers from the Perelman School of Medicine at the University of Pennsylvania instead studied six healthy young male volunteers to collect physiological information as they went about their normal daily lives. The Penn study measured thousands of physiological indicators and used wearable devices and smart phone apps to gather part of it.
"We integrated data from remote sensors, wearables, and physiological samples to see how feasible it would be to detect an oscillatory phenotype, the chronobiome, of an individual, despite the 'noise' of everyday life," said first author Carsten Skarke, MD, a research assistant professor of Medicine.
"We essentially defined each participant's 'chronobiome' -a collection of their individual physiological traits in a 24-hour rhythmic pattern – as a reflection of their time-dependent deep phenotype under free-ranging conditions," said senior author Garret FitzGerald, MD, director of the Institute for Translational Medicine and Therapeutics, who coined the term.
The molecular circadian clock coordinates the body's rhythms fine-tuned by environmental cues, such as light, to the 24-hour solar cycle. A master clock in the brain communicates that control to molecular clocks in peripheral tissues. In humans, many aspects of physiology, including body temperature, levels of blood glucose, insulin, hormones, and neurotransmitters vary on a daily cycle.
In their study, the majority – 62 percent – of sensor readouts showed time-specific variability, including the expected variation in blood pressure, heart rate, and cortisol. "We saw that several clock-determined traits such as blood pressure and cortisol were consistent with time-dependent patterns in the volunteers' physical activity, mobility, communication, and environmental cues such as exposure to ambient light," Skarke said.
In humans, the incidence or severity of many diseases, such as asthma, myocardial infarction, stroke, and depression exhibit diurnal variation. Similarly, the levels of molecular targets of many drugs oscillate, as do enzymes and transporters relevant to drug metabolism.
"We need a baseline of healthy chronobiomes to be able to detect and interpret discordant data collected from patients before we can think of tailoring drug regimens for time-specific diseases," Skarke said. Despite the long-recognized, time-dependent variation in the effectiveness of many commonly used drugs, there has been little use of chronotherapy in clinical practice.
For this pilot study, six young, urban, male professionals were fitted with a watch-like wearable device to measure their daily activity levels and light exposure. Their smartphones were outfitted with an app that relayed the number of texts and calls each made every day while phone-based GPS kept track of their mobility. They also came to the lab for two 48-hour sessions in which their saliva, blood, and other body fluids were sampled for molecular biomarkers. During this time, their ambulatory blood pressures were recorded, and they kept track of their dietary intake in calories by taking images of their meals and snacks before and after consumption, which was uploaded to nutritionists to calculate offsite.
"Now, we have proof-of-concept to scale up our work to characterize the human chronobiome in a larger population of 'free-living' people," FitzGerald said.
The researchers emphasize the utility of a detailed characterization of the chronobiome and how it might be altered under stressful conditions — bacterial infection, for example.
"We plan to scale up to 200 volunteers of both sexes and different ages, measure change over seasons, and study what changes are evoked in the chronobiome as a response to metabolic, cardiovascular, and inflammatory stressors, as well as extend our approach to study diseases associated with disrupted clocks," said Skarke. This approach is a necessary prelude to detect differences in the chronobiome to ultimately find therapeutic value in patients with circadian time-dependent diseases, such as non-dipping hypertension, nocturnal asthma, depression, and night-eating syndrome.
The Penn team now has online pilot studies with surgical, HIV, heart disease, and asthma patients, as well as shift workers.
Skarke and FitzGerald recognize the potential of chronotherapy becoming integrated into clinical care in many ways. For instance, if it's assumed that a drug should be taken at bedtime, what does that mean for an individual chronotype? Should it be a different regimen for morning larks versus night owls? They propose that patients' chronobiomes could be characterized using a wearable, their cell phone, and biomarkers from their blood, urine, saliva, and feces. Then a drug could be dosed according to an individual's chronobiome. Indeed, earlier research at Penn identified that a majority of prescription drugs act on enzymes and proteins that oscillate over 24 hours.
###
Other members of the team include Nicholas F. Lahens, PhD; Seth D. Rhoades, PhD; Amy Campbell, PhD; Kyle Bittinger, PhD; Aubrey Bailey, PhD; Christian Hoffmann, PhD; Randal S. Olson, PhD; Lihong Chen, MD, PhD; Guangrui Yang, MD, PhD; Thomas S. Price, PhD; Jason H. Moore, PhD; Frederic D. Bushman, PhD; Casey S. Greene, PhD; Gregory R. Grant, PhD; and Aalim M. Weljie, PhD, all from Penn.
This work was funded by a Clinical Research Program Award (12CRP11920045); Great Rivers Affiliate, American Heart Association; Feodor-Lynen Research Award, Alexander von Humboldt-Foundation, Bonn, Germany; and the National Institutes of Health (1P30 ES013508-05, LM00901, UL1TR000003).
Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $6.7 billion enterprise.
The Perelman School of Medicine has been ranked among the top five medical schools in the United States for the past 20 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $392 million awarded in the 2016 fiscal year.
The University of Pennsylvania Health System's patient care facilities include: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center — which are recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report — Chester County Hospital; Lancaster General Health; Penn Wissahickon Hospice; and Pennsylvania Hospital — the nation's first hospital, founded in 1751. Additional affiliated inpatient care facilities and services throughout the Philadelphia region include Good Shepherd Penn Partners, a partnership between Good Shepherd Rehabilitation Network and Penn Medicine.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2016, Penn Medicine provided $393 million to benefit our community.
Media Contact
Karen Kreeger
[email protected]
215-459-0544
@PennMedNews
http://www.uphs.upenn.edu/news/