Credit: University of Southampton
Researchers at the University of Southampton have engineered cells with a 'built-in genetic circuit' that produces a molecule that inhibits the ability of tumours to survive and grow in their low oxygen environment.
The genetic circuit produces the machinery necessary for the production of a compound that inhibits a protein which has a significant and critical role in the growth and survival of cancer cells. This results in the cancer cells being unable to survive in the low oxygen, low nutrient tumour micro-environment.
As tumours develop and grow, they rapidly outstrip the supply of oxygen delivered by existing blood vessels. This results in cancer cells needing to adapt to low oxygen environment.
To enable them to survive, adapt and grow in the low-oxygen or 'hypoxic' environments, tumours contain increased levels of a protein called Hypoxia-inducible factor 1 (HIF-1). HIF-1 senses reduced oxygen levels and triggers many changes in cellular function, including a changed metabolism and sending signals for the formation of new blood vessels. It is thought that tumours primarily hijack the function of this protein (HIF-1) to survival and grow.
Professor Ali Tavassoli, who led the study with colleague Dr. Ishna Mistry, explains: "In an effort to better understand the role of HIF-1 in cancer, and to demonstrate the potential for inhibiting this protein in cancer therapy, we engineered a human cell line with an additional genetic circuit that produces the HIF-1 inhibiting molecule when placed in a hypoxic environment.
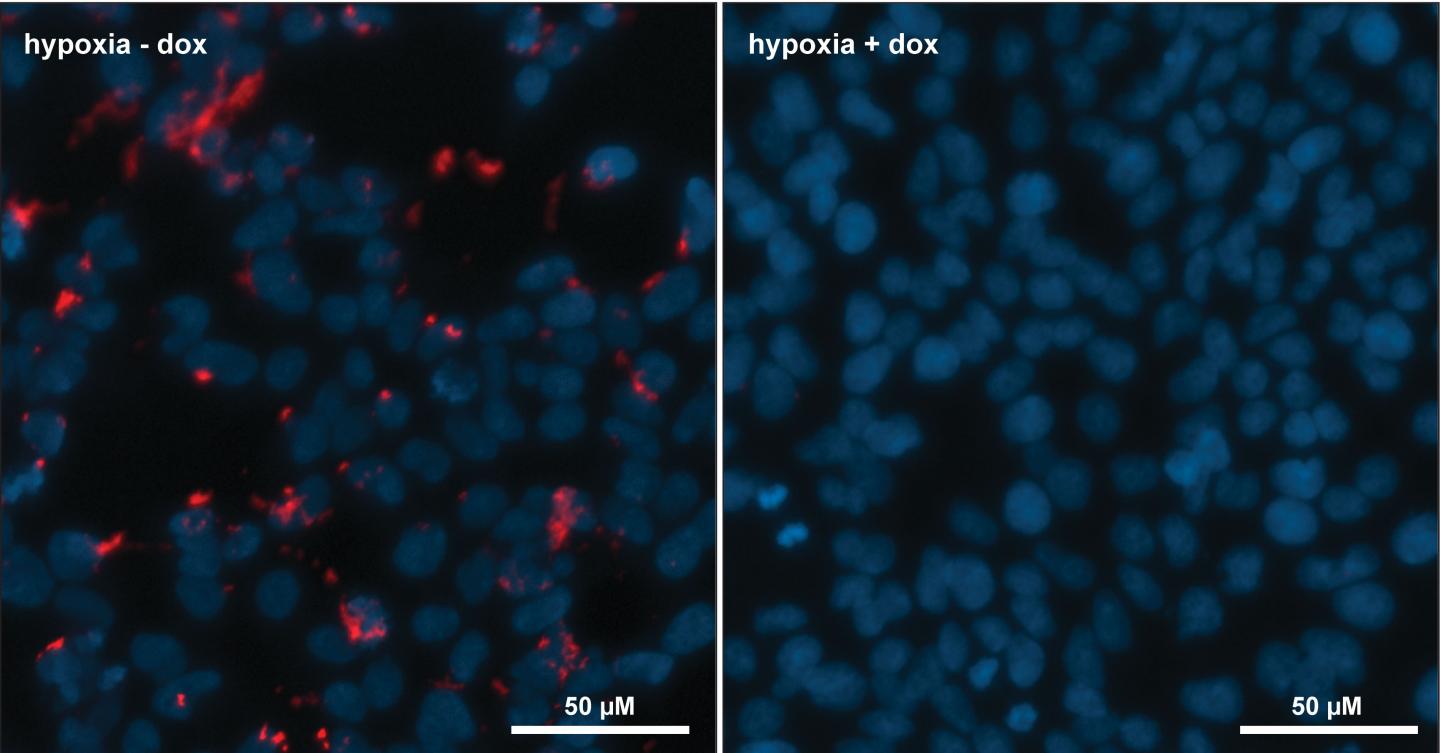
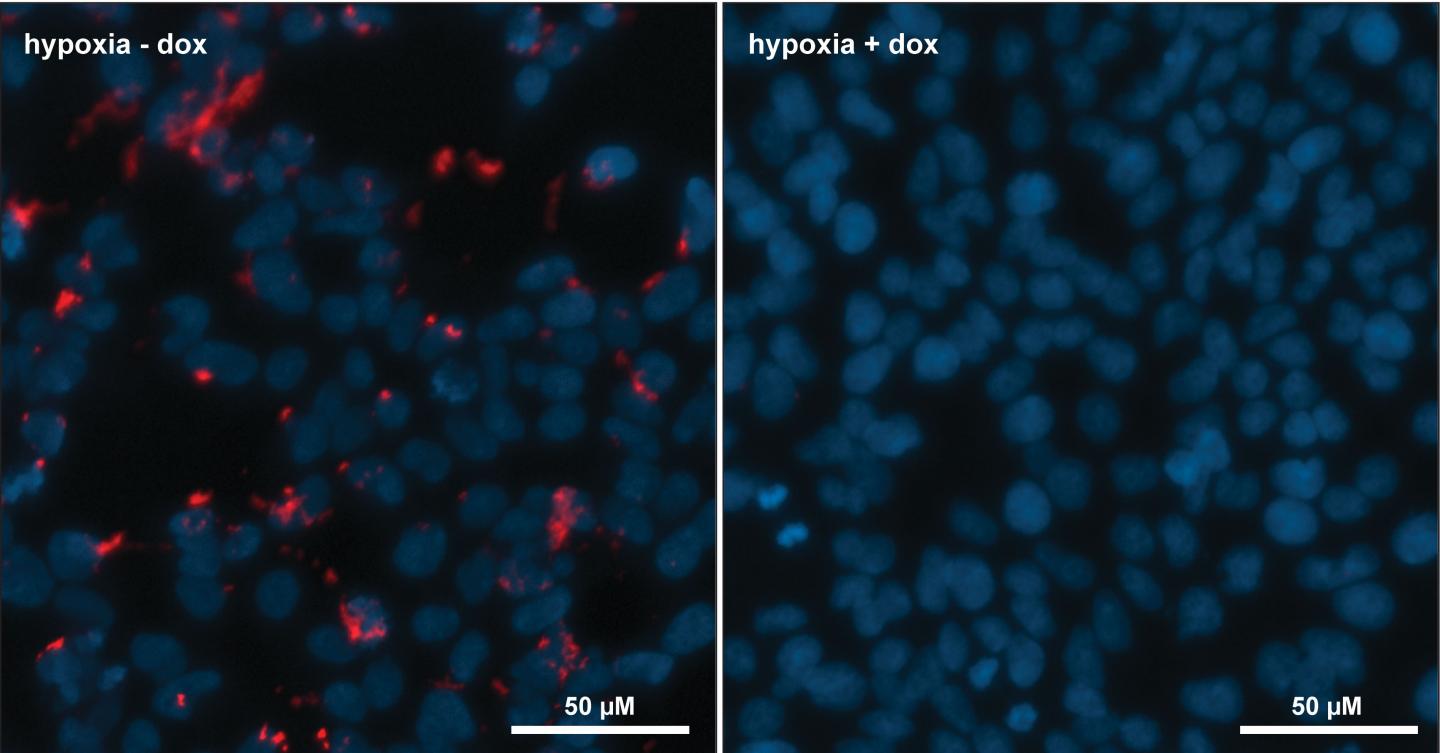
"We've been able to show that the engineered cells produce the HIF-1 inhibitor, and this molecule goes on to inhibit HIF-1 function in cells, limiting the ability of these cells to survive and grow in a nutrient-limited environment as expected.
"In a wider sense, we have given these engineered cells the ability to fight back – to stop a key protein from functioning in cancer cells. This opens up the possibility for the production and use of sentinel circuits, which produce other bioactive compounds in response to environmental or cellular changes, to target a range of diseases including cancer."
The genetic circuit is incorporated onto the chromosome of a human cell line, which encodes the protein machinery required for the production of their cyclic peptide HIF-1 inhibitor. The production of the HIF-1 inhibitor occurs in response to hypoxia in these cells. The research team demonstrated that even when produced directly in cells, this molecule still prevents the HIF-1 signalling and the associated adaptation to hypoxia in these cells.
The next step for the researchers is to demonstrate the viability of this approach to the production and delivery of an anticancer molecule in a whole tumour model system.
Professor Tavassoli adds: "The main application for this work is that it eliminates the need for the synthesis of our inhibitor, so that biologists conducting research into HIF function can easily access our molecule and hopefully discover more about the role of HIF-1 in cancer. This will also let us understand whether inhibiting HIF-1 function alone is enough to block cancer growth in relevant models. Another interesting aspect to the work is that it demonstrates the possibility of adding new machinery to human cells to enable them to make therapeutic agents in response to disease signals."
The study, which was funded by Cancer Research UK and the Engineering and Physical Sciences Research Council, is published in the journal ACS Synthetic Biology.
###
Media Contact
Becky Attwood
[email protected]
@unisouthampton
http://www.southampton.ac.uk/
############
Story Source: Materials provided by Scienmag