Credit: (Purdue University image/S. Saif Hasan)
February 16, 2017 – WEST LAFAYETTE, Ind. — Researchers have determined the structure of a human antibody bound to the Zika virus, revealing details about how the antibody interferes with the infection mechanism — findings that could aid in development of antiviral medications.
The new findings also suggest the antibody might be especially effective because a lower concentration than expected is needed to inhibit a key mechanism of infection, making it more potent than previous antibodies studied. The research was performed by a team from Purdue University, Vanderbilt University Medical Center and the Washington University School of Medicine.
The human antibody was isolated by the Vanderbilt and Washington University researchers, who reported their findings earlier this year. Those findings showed that the antibody, which was isolated from a person previously infected with Zika virus, neutralizes Zika strains that belong to African, Asian and American lineages and is able to reduce fetal infection and death in mice.
"However, until now what remained unknown was the mechanism of neutralization of Zika infection by the antibody and the structural basis for neutralization," said Michael Rossmann, Purdue's Hanley Distinguished Professor of Biological Sciences.
The findings are being reported today (March 16) in the journal Nature Communications.
The research team was led by Rossmann and Richard Kuhn, both professors in Purdue's Department of Biological Sciences, and senior postdoctoral scientist S. Saif Hasan. Research to isolate the antibody was led by James E. Crowe Jr., a professor of pediatrics, pathology, microbiology and immunology at Vanderbilt, and Michael S. Diamond, the Herbert S. Gasser Professor at Washington University. A YouTube video is available at https://youtu.be/DQast8epdOw.
Zika belongs to a family of viruses called flaviviruses, which includes dengue, West Nile, yellow fever, Japanese encephalitis and tick-borne encephalitic viruses.
In the new findings, researchers determined the combined three-dimensional structure of the Zika virus while attached to a key binding site on the antibody known as the antigen binding fragment, or a Fab molecule.
"It has potential to be a therapeutic neutralizing human antibody" said Kuhn, director of the Purdue Institute of Inflammation, Immunology and Infectious Disease (PI4D).
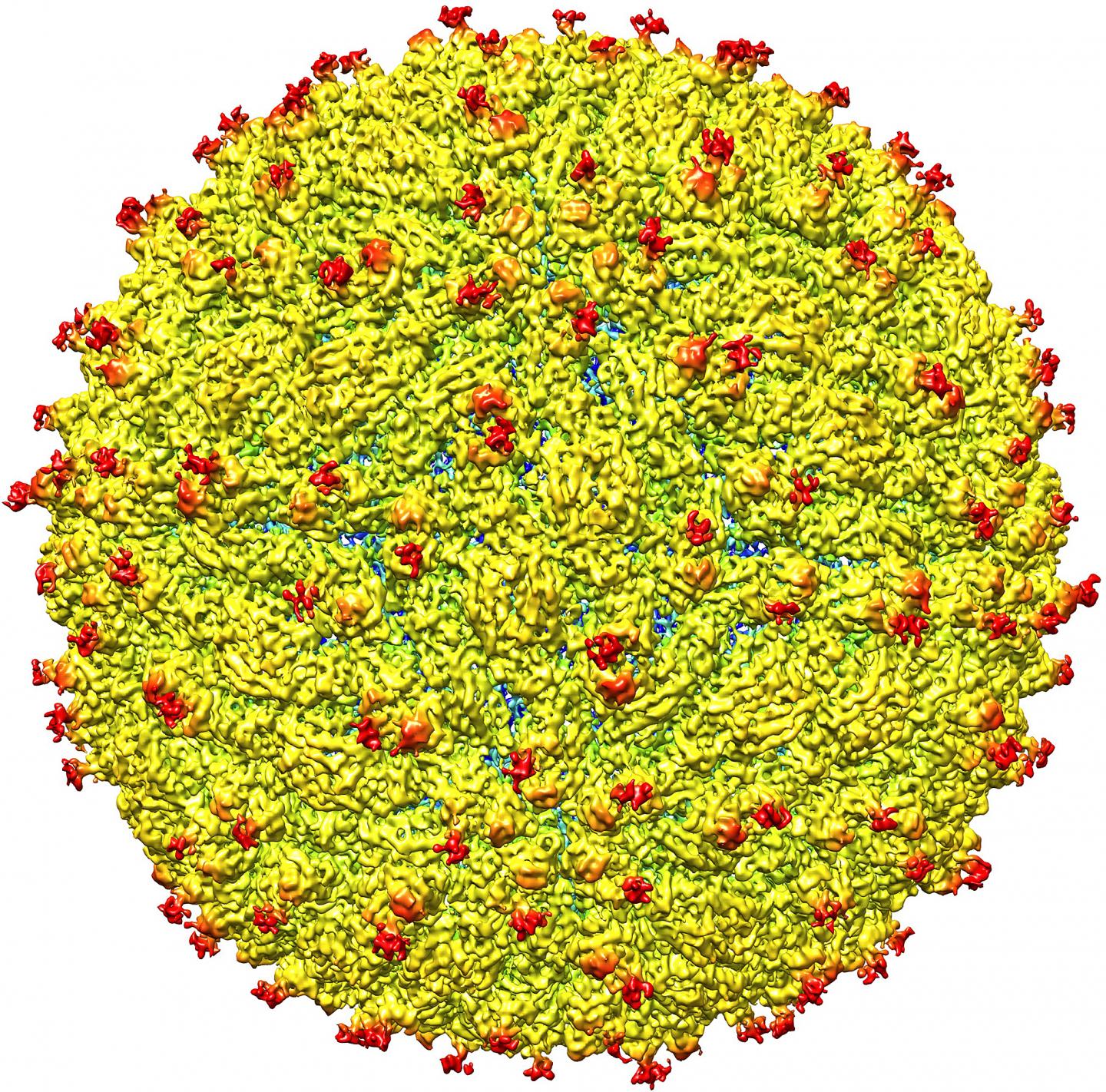
The genome of the Zika virus is housed inside a protective shell that includes 60 repeating units, each containing three envelope proteins, or E proteins. As the virus attaches to a host cell's outer membrane a difference in pH, or acidity, in the membrane causes these "trimers" to expose "fusion peptides," leading to the transfer of the viral RNA genome, a step critical to infection. The new findings show the antibody's binding to Zika inhibits this pH-triggering mechanism, neutralizing the virus by "cross-linking" the E proteins, tying them up and preventing their reorganization into "fusogenic" trimers.
"This hypothesis is supported by pre- and post-neutralization assays of Zika infection, showing the antibody is able to significantly inhibit infection," Rossmann said. "This approach should provide broad-range protection against virtually all strains of Zika."
Moreover, considering that the surface of Zika is made of 60 copies of three E proteins, it would be expected that 180 copies of the antibody's Fab molecules would be needed for neutralization.
"However, one antibody binds for six E proteins, so only 30 are needed," Hasan said. "Therefore, you don't need a high concentration of antibodies to achieve neutralization."
The findings primarily will aid in the development of antiviral drugs but also will help researchers identify important sites on the virus for human antibodies to hook onto, which could be useful in developing vaccines down the road, Kuhn said.
The researchers determined the structure at a resolution of 6.2 Ångstroms using a technique called cryo-electron microscopy.
The Zika virus has been associated with a birth defect called microcephaly that causes brain damage and an abnormally small head in babies born to mothers infected during pregnancy. The virus also has been associated with the autoimmune disease Guillain-Barré syndrome, which can lead to temporary paralysis.
"Given the severity of the symptoms caused by Zika infection in humans, it is crucial to understand the immune response elicited by the infection to develop neutralizing anti-Zika therapies," Rossmann said. "In contrast to other flaviviruses that are spread mainly by insects, recent evidence suggests that Zika can be transmitted sexually and from mother to child in addition to transmission by mosquitoes."
The first major outbreak of the Zika virus was recorded in 2007 in Micronesia and then in 2013-14 in Oceania. The latest outbreak, which started in Brazil in 2014-15, has spread to other countries in South America, North America and the Caribbean. Four cases of fetal deformities were reported in December 2016 in New York City.
###
The paper was authored by Purdue senior postdoctoral scientist S. Saif Hasan; Purdue staff scientist Andrew Miller; Vanderbilt research scientist Gopal Sapparapu; Estefania Fernandez, a pre-doctoral student at the Washington University School of Medicine; Purdue assistant research scientist Thomas Klose; Purdue postdoctoral research associates Feng Long, Andrei Fokine and Jason C. Porta; Wen Jiang, a Purdue professor of biological sciences and chemistry; Diamond; Crowe; Kuhn; and Rossmann.
Future research will include work to study other antibodies.
"Different antibodies neutralize the virus in different ways, so we want to understand these mechanisms," Rossmann said.
The research was funded by the National Institutes of Health.
Writer: Emil Venere, 765-494-4709, [email protected]
Sources: Richard Kuhn 765-494-1164, [email protected] Michael Rossmann 765-494-4911, [email protected]
PHOTO CAPTION:
Richard Kuhn, at left, a professor in Purdue University's Department of Biological Sciences, and Michael Rossmann, Hanley Distinguished Professor of Biological Sciences, have led research revealing details about how a human antibody to the Zika virus interferes with infection. (Purdue University photo/Mark Simons)
A publication-quality image is available at https://news.uns.purdue.edu/images/2016/kuhn-rossmann.jpg
PHOTO CAPTION:
This color-coded image depicts the surface view of the Zika virus bound to fragments of a human antibody, shown as red knobs. Researchers have determined the structure of the antibody bound to the virus, findings that could aid in development of antiviral medications. (Purdue University image/S. Saif Hasan) A publication-quality image is available at https://news.uns.purdue.edu/images/2016/rossmann-zika.jpg
ABSTRACT
A Human Antibody Against Zika Virus Crosslinks the E Protein to Prevent Infection
S. Saif Hasan1, Andrew Miller1, Gopal Sapparapu2,3, Estefania Fernandez4, Thomas Klose1, 2 Feng Long1, Andrei Fokine1, Jason C. Porta1, Wen Jiang1,5, Michael S. Diamond4,7,8,9, James E. Crowe, Jr. 2,3,6, Richard J. Kuhn1,5,*, Michael G. Rossmann1,5,*
1Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA
2Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN 37232, USA
3The Vanderbilt Vaccine Center, Vanderbilt University Medical Center
4Department of Pathology & Immunology, Washington University School of Medicine, St Louis, 10 MO 63110, USA
5Markey Center for Structural Biology and Purdue Institute for Inflammation, Immunology and Infectious Disease, Purdue Universityv
6Department of Pathology, Microbiology and Immunology, Vanderbilt University
7Department of Medicine, Washington University School of Medicine
8Department of Molecular Microbiology, Washington University School of Medicine
9Center for Human Immunology and Immunotherapy Programs, Washington University School of Medicine
*Co-corresponding authors. Address correspondence to: Richard J. Kuhn, Hockmeyer Hall of Structural Biology, 240 S. Martin Jischke Drive, West Lafayette IN 47907, USA. [email protected] (765)-494-1164 and Michael G. Rossmann, Hockmeyer Hall of Structural Biology, 240 S. Martin Jischke Drive, West Lafayette IN 47907, USA. [email protected] (765)-494-4911
The recent Zika virus (ZIKV) epidemic has been linked to unusual and severe clinical manifestations including microcephaly in fetuses of infected pregnant women 30 and Guillain-Barré syndrome in adults. Neutralizing antibodies present a possible therapeutic approach to prevent and control ZIKV infection. Here we present a 6.2 Å resolution three-dimensional cryo-electron microscopy (cryoEM) structure of an infectious ZIKV (strain H/PF/2013, French Polynesia) in complex with the Fab fragment of a highly therapeutic and neutralizing human monoclonal antibody, ZIKV-117. The antibody had been shown to prevent fetal infection and demise in mice. The structure shows that ZIKV-117 Fabs cross-link the monomers within the surface E glycoprotein dimers as well as between neighboring dimers, thus preventing the reorganization of E protein monomers into fusogenic trimers in the acidic environment of endosomes.
Note to Journalists: A copy of the research paper is available from the Nature Press Office at [email protected] or from Emil Venere, Purdue News Service, at 765-494-4709, [email protected] A YouTube video is available at https://youtu.be/DQast8epdOw
Media Contact
emil venere
[email protected]
765-494-4709
@PurdueUnivNews
http://www.purdue.edu/