Genetic expression, often leading to protein synthesis, requires a complex coordination of molecular machinery across several stages. A vital step in protein-coding gene expression is messenger RNA (mRNA) export, which involves shuttling mature mRNAs from the cell’s nucleus to the cytoplasm.
Credit: Seiji Masuda from Kindai University
Genetic expression, often leading to protein synthesis, requires a complex coordination of molecular machinery across several stages. A vital step in protein-coding gene expression is messenger RNA (mRNA) export, which involves shuttling mature mRNAs from the cell’s nucleus to the cytoplasm.
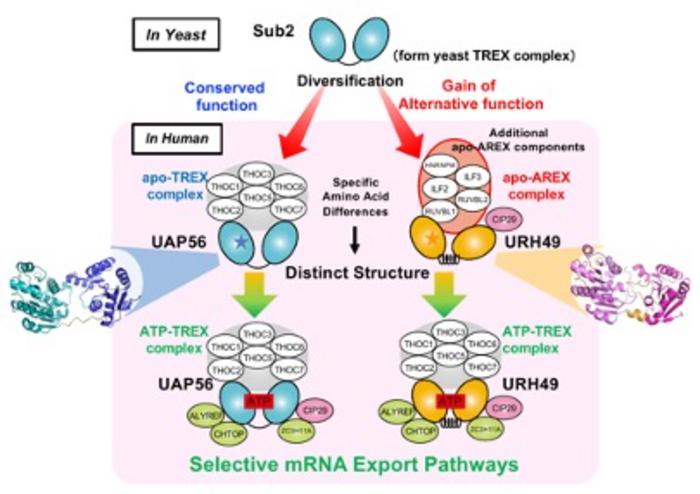
The mRNA export process relies on mRNA–protein complex formation, with the evolutionary conserved ATP-bound TREX complex playing a pivotal role. Among its components, the RNA helicase UAP56 is perhaps the most important one during its assembly. Not only does UAP56 participates during mRNA splicing in some transcripts, but it also recruits necessary proteins for export upon ATP binding. It is noteworthy that the ATP-unbound form of the TREX complex is termed “apo-TREX,” while the ATP-bound form is “ATP-TREX.”
Interestingly, mammals share a paralogue of UAP56, an almost identical gene known as URH49. Scientists demonstrated that URH49 also forms an mRNA–protein complex, called “apo-AREX,” which significantly differs in composition from apo-TREX. However, upon binding to ATP, apo-AREX transforms into a complex nearly identical to ATP-TREX. They are so similar that they are both called APT-TREX regardless of whether the precursor was apo-AREX or apo-TREX. Despite studies showing that UAP56 and URH49 complexes export different subsets of mRNA, little is known about the structural and functional differences between the apo-complexes formed by these distinct RNA helicases.
In a recent study published in Nature Communications on 15 January 2024, a research team from Japan, led by Professor Seiji Masuda from Kindai University in Japan, aimed to address these knowledge gaps. Their paper was co-authored by Dr. Ken-ichi Fujita of Fujita Health University and the National Cancer Center Research Institute, Dr. Masaki Kojima of Tokyo University of Pharmacy and Life Sciences, Dr. Bunzo Mikami of Kyoto University, and Dr. Akila Mayeda of Fujita Health University et al.
First, the researchers conducted immunoprecipitation experiments in genetically modified cells expressing immunotagged UAP56 and URH49 to identify the components of the apo-complexes. They identified several factors that are part of apo-AREX, including RUVBL1, RUVBL2, ILF2, and ILF3, which are not present in ATP-TREX complexes.
Subsequently, the team investigated the functional significance of these factors. Through knockdown experiments using short interfering RNAs, they found that depleting any of the factors resulted in mature mRNA accumulation in the cell nucleus. Additionally, there was an increase in the expression of UAP56 targets as a compensatory mechanism. These findings suggest the identified factors play a crucial role in the apo-AREX complex, ultimately regulating URH49-specific mRNA transport.
Next, the researchers focused on the structural differences between apo-AREX and apo-TREX complexes. “We hypothesized that differences in a specific regions between UAP56 and URH49 are important to form their distinct apo-complexes,” explains Prof. Masuda. “To identify the regions, we created plasmids expressing various mutants of UAP56 and URH49, swapping different parts.” On top of this, they conducted several additional experiments involving characterizing the crystal structure of URH49 using X-ray diffraction and studying the differences between the ATP-bound and ADP-bound TREX complexes.
Interestingly, the team discovered that a change in a single amino acid between UAP56 and URH49 is enough to determine the formation of either apo-TREX or apo-AREX complexes. By examining the Sub2 gene in yeast, the ancestor gene of UAP56, the researchers concluded that the apo-structure of UAP56 was evolutionarily conserved, whereas that of URH49 emerged during evolution as a point mutation that ultimately resulted in new functions.
Overall, this study has shed some light on both the similarities and differences between two important complexes formed by RNA helicases. This understanding is vital for comprehending their distinct roles during mRNA processing and export. “Components of the TREX and AREX complexes appear to be overexpressed in a variety of cancer cells. Maintaining adequate expression of these components could be important to keep cells healthy, and their aberrant expression may also be a cause of cancer,” comments Prof. Masuda.
Hopefully, continued exploration of these protein complexes will help scientists not only understand the evolution of eukaryotes, but also lead to new diagnostic and therapeutic strategies for challenging diseases.
Journal
Nature Communications
DOI
10.1038/s41467-023-44217-8
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Structural differences between the closely related RNA helicases, UAP56 and URH49, fashion distinct functional apo-complexes
Article Publication Date
15-Jan-2024
COI Statement
The authors declare no competing interests




