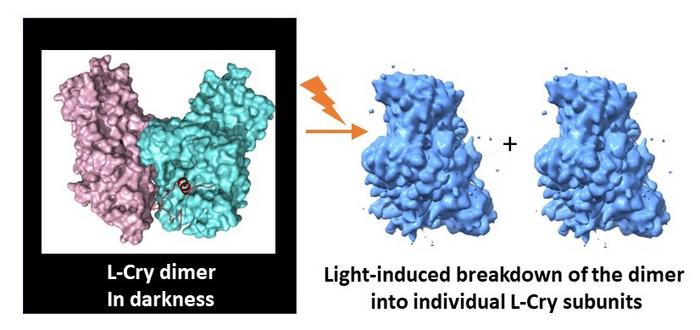
In a recent publication in Nature Communications, a joint research team of Johannes Gutenberg University Mainz (JGU), the University of Cologne, and the University of Oldenburg has presented their findings on the functioning of an atypical cryptochrome protein (Cry). These proteins are found in a variety of organisms, and they are often involved in light-controlled biological processes. The marine bristle worm Platynereis dumerilii, for example, employs a special Cry protein designated L-Cry to distinguish between sunlight and moonlight as well as between different moon phases. This is essential for the worms to synchronize their reproduction to the full moon phase via an inner monthly calendar, also called circalunar clock. The researchers in Cologne used the cryo-electron microscopy platform of their university to visualize the three-dimensional structure of the L-Cry protein under different light conditions. The results of these structural analyses together with those of the biochemical investigations undertaken primarily at Mainz University revealed that, in the dark, L-Cry adopts a so-called dimer arrangement consisting of two subunits linked by a stable connection, while under intensive sunlight-like illumination it disassembles into its subunits or monomers.
Credit: ill./©: Eva Wolf and Hong Ha Vu
In a recent publication in Nature Communications, a joint research team of Johannes Gutenberg University Mainz (JGU), the University of Cologne, and the University of Oldenburg has presented their findings on the functioning of an atypical cryptochrome protein (Cry). These proteins are found in a variety of organisms, and they are often involved in light-controlled biological processes. The marine bristle worm Platynereis dumerilii, for example, employs a special Cry protein designated L-Cry to distinguish between sunlight and moonlight as well as between different moon phases. This is essential for the worms to synchronize their reproduction to the full moon phase via an inner monthly calendar, also called circalunar clock. The researchers in Cologne used the cryo-electron microscopy platform of their university to visualize the three-dimensional structure of the L-Cry protein under different light conditions. The results of these structural analyses together with those of the biochemical investigations undertaken primarily at Mainz University revealed that, in the dark, L-Cry adopts a so-called dimer arrangement consisting of two subunits linked by a stable connection, while under intensive sunlight-like illumination it disassembles into its subunits or monomers.
It is not only the spatial arrangement of the two subunits in the dark that is unusual and corresponds to an arrangement not previously observed in other Cry proteins. The direction of light-induced changes is also unusual, since for other Cry proteins only the reverse process has been described, i.e., from monomer arrangements in the dark to dimer or higher oligomer arrangements in the light. The research team was also able to identify the main structural features in the protein that are important for this unusual behavior. Furthermore, knowledge of the three-dimensional structure enabled the researchers to introduce targeted mutations in the L-Cry protein to further characterize its functioning as a photoreceptor.
“Our findings could explain how L-Cry manages to distinguish between sunlight and moonlight: Intense sunlight always activates both subunits of the dimer simultaneously, which initiates its breakdown into individual subunits. The significantly weaker moonlight, however, statistically only activates one of two subunits,” explained Professor Eva Wolf of the JGU Institute of Molecular Physiology, who led the study at Mainz University. The results of the study highlight the uniqueness of L-Cry among the highly diverse Cry proteins with their wide range of functions. They are also thought, for example, to be sensor proteins in the perception of the Earth’s magnetic field in birds.
First steps of decoding the molecular processes of the circalunar clock
“Working with light-sensitive proteins is always a challenge,” said Hong Ha Vu, a doctoral candidate in the JGU research group of Professor Eva Wolf and a major contributor to the study. “When preparing the L-Cry proteins for analysis, we need to carry out all experimental processes in the dark or under specifically defined red light conditions to prevent unintentional pre-activation of these very light-sensitive proteins. For the functional characterization of L-Cry, it is also necessary to use lighting conditions similar to underwater natural sunlight and moonlight illumination of the kind that the bristle worms encounter in their natural habitat. Only then we can compare the specific properties of L-Cry in its role as a sunlight and moonlight receptor with those of other cryptochromes.”
And Professor Eva Wolf added: “Our investigations have provided important new insights into how this most unusual sunlight and moonlight receptor works. Furthermore, our structural and molecular mechanistic insights into L-Cry’s function have opened up future avenues of research that should help us better understand the still largely unknown molecular processes involved in synchronization of the circalunar clock with the moon phases.”
Journal
Nature Communications
DOI
10.1038/s41467-023-42708-2
Article Title
A marine cryptochrome with an inverse photo-oligomerization mechanism
Article Publication Date
30-Oct-2023




