(Santa Barbara, Calif.) — In work conducted both at UC Santa Barbara and the Physics of Life Excellence Cluster of TU Dresden, biophysicist Otger Campàs and his research group have found that cell nuclei control the architecture and mechanics of eye and brain tissues during embryonic development. These results add a new role for the cell’s nucleus in tissue organization, well beyond its established role in genetic regulation.
(Santa Barbara, Calif.) — In work conducted both at UC Santa Barbara and the Physics of Life Excellence Cluster of TU Dresden, biophysicist Otger Campàs and his research group have found that cell nuclei control the architecture and mechanics of eye and brain tissues during embryonic development. These results add a new role for the cell’s nucleus in tissue organization, well beyond its established role in genetic regulation.
“We were measuring tissue stiffness in the zebrafish retina, and realized that it depended on the packing of nuclei. This was totally unexpected because tissue mechanics is believed to depend on cell surface interactions, but not organelles inside cells,” said Campàs, now professor and the chair of tissue dynamics at the Cluster of Excellence Physics of Life at TU Dresden, where he also serves as managing director. This research, published in the journal Nature Materials, represents an unexplored avenue to understand how cells orchestrate embryonic development.
The Hidden Architect
Inside each cell, individual structures known as organelles perform key functions, but how these organelles contribute to the formation of tissues and organs is unknown. Like factories or roads in cities, myriad organelles perform tasks inside cells for them to properly function. Because they are confined within cells, organelles were not believed to play a direct role in building organs during embryogenesis. Until now.
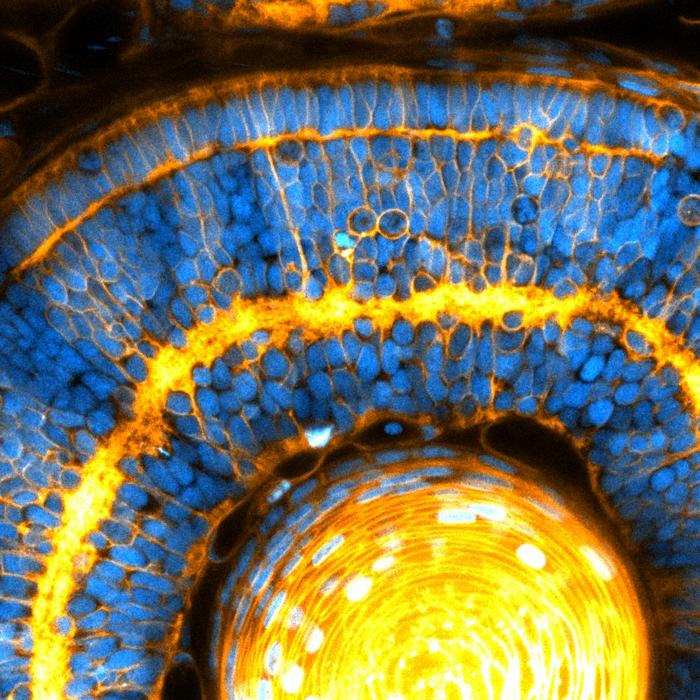
The cell’s nucleus is an organelle known for processing information in cells, with genes turning on and off depending on the signals received. However, the nucleus is also the largest and stiffest organelle in cells, and could affect the physical structure of the tissue in addition to processing information. Fascinated with how the nucleus could play a role in tissue formation, Campàs decided to study the role of nuclei in the formation of organs.
Previous pioneering work by his group had found that cell collectives acted like a foam during development that could be either jammed to “freeze” the tissue architecture and set its shape, or ‘melted’ to allow tissues to flow and shape them.
“By extending the Active Foam Model, we identified a new mode of solid-to-fluid transition, governed by the relative nucleus and cell sizes,” said co-lead author Sangwoo Kim.
When the authors investigated size of the nucleus compared to that of cells in eye and brain tissues in both experimental and theoretical settings, they found that if the nucleus was taking much of the cell space, then the tissue stiffness was directly controlled by the nucleus. Moreover, they also found that when the nuclei packed so strongly, they ordered the cells into nearly crystalline arrays.
“When the nuclei start to interact mechanically, both tissue mechanics and cellular ordering are not dictated by the cell surface, but rather controlled by the nucleus itself,” Campàs said. “This is an organelle determining the stiffness of the entire tissue.” Their study challenges the status quo, revealing a new role for nuclei in the control of tissue organization and mechanics.
To explore how the size of the cell’s nucleus affects organ formation, the researchers employed zebrafish. These vertebrates are an invaluable model to explore developmental questions, as they are fully transparent during their embryonic stages and mature rapidly, allowing the visualization of organ formation in 3D.
“We therefore conducted structural measurements and cell movement quantifications, focusing on the developing retina and brain of zebrafish,” said co-lead author Rana Amini.
With these measurements, the authors demonstrated that changes in cell and nuclear sizes during key stages of development ‘jam’ the nuclei into place, as they become tightly surrounded by their neighbours. During this transition, nuclei fit neatly together, like coffee beans in a jar, and this organization may be important for the eye to function. In our eyes, the packing of cells seems very structured, often displaying a very regular, “crystalline” order necessary to process visual cues. In zebrafish it is no different, and the crystalline order of cells appears to be a result of the jamming of nuclei as the eye develops.
Beyond the eye, the team also found that brain tissues become nuclear jammed, revealing a new role for the nucleus to control the architecture of several neural tissues. This work also highlights a potential role for defects at the nuclear level to cause diseases associated with impaired tissue architecture. This new piece of the puzzle, puts us one step closer to understanding how cells build organs during embryonic development.
Journal
Nature Materials
Article Title
A nuclear jamming transition in vertebrate organogenesis
Article Publication Date
12-Aug-2024