In a groundbreaking study emerging from Kyushu University, researchers have unveiled a previously unrecognized role for the histone modification H3K4me3 in the maturation and developmental competence of mouse oocytes. Published in the prestigious Journal of Biological Chemistry, this work elucidates how H3K4me3, a trimethylation mark found on histone H3 at lysine 4, is intricately involved in stabilizing chromosome positioning and spindle architecture during the metaphase II (MII) stage, a critical juncture preceding fertilization. The discovery challenges long-standing assumptions about this epigenetic mark, traditionally linked to active gene transcription, by revealing its essential structural function in transcriptionally silent oocytes.
Histones, the protein complexes around which DNA winds, are subject to a dynamic network of post-translational modifications that modulate access to genetic information. Among these, methylation of H3K4 is often associated with gene activation, appearing prominently in euchromatic regions where transcription occurs. However, the MII oocyte represents a unique cellular context; it is arrested in meiosis with transcriptional activity largely halted, raising compelling questions about the functional significance of high H3K4me3 abundance at this stage. The Kyushu team, spearheaded by Professor Kei Miyamoto, pursued these questions with an integrative approach combining advanced imaging and molecular manipulation to localize and interrogate H3K4me3 function in mouse oocytes.
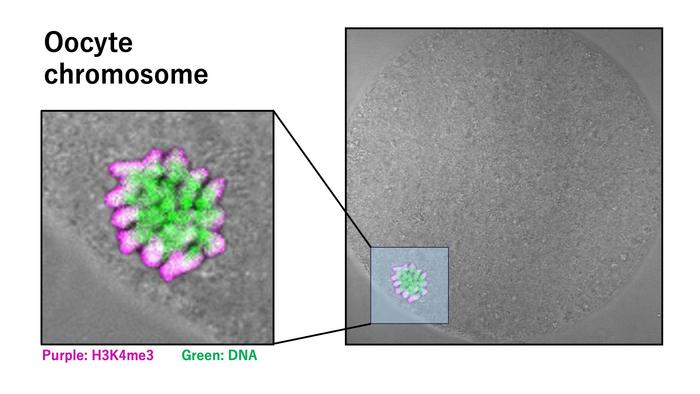
High-resolution immunofluorescence microscopy revealed a remarkable asymmetric accumulation of H3K4me3 in MII oocytes. This mark was heavily enriched on the side of chromosomes facing the oocyte cortex, particularly highlighting the X chromosomes. Such localized distribution was absent from other chromosomal regions, indicating chromosome-specific regulation. The actin cap, a cytoskeletal structure intimately involved in positioning the meiotic spindle and chromosomes, was identified as a likely facilitator of this polarized histone modification pattern. This spatial organization hints at a sophisticated interplay between epigenetic regulation and cytoskeletal dynamics in orchestrating meiotic progression.
.adsslot_sUXayMiZJB{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_sUXayMiZJB{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_sUXayMiZJB{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Testing the functional ramifications of H3K4me3’s localization, researchers employed targeted enzymatic strategies to selectively remove this modification in MII oocytes. The depletion of H3K4me3 led to conspicuous spindle abnormalities, notably a reduction in spindle length and compromised integrity. Since the spindle apparatus ensures accurate segregation of chromosomes during cell division, its destabilization portends significant developmental consequences. These structural perturbations were corroborated by live imaging and quantitative assessments, firmly establishing H3K4me3 as a vital stabilizing factor beyond its canonical role in gene expression.
Moreover, the researchers uncovered an age-associated decline in H3K4me3 levels within oocytes, linking epigenetic alterations to reproductive aging. This observation aligns with known decreases in oocyte quality with advanced maternal age and implicates the erosion of H3K4me3 as a contributing molecular mechanism. By connecting histone modification deficiencies to age-related fertility decline, the study opens avenues for therapeutic intervention aimed at preserving or restoring epigenetic integrity in aging female gametes.
Professor Miyamoto emphasizes the transformative potential of these findings, stating that uncovering a non-transcriptional role for such a well-studied histone modification paves the way to novel fertility treatments. Manipulating H3K4me3 dynamics could offer strategies to combat infertility and reduce miscarriage rates by stabilizing oocyte structures critical for chromosome segregation. Future research will delve deeper into the molecular pathways that mediate H3K4me3 localization and function, potentially revealing targets for pharmacological modulation.
At the mechanistic level, questions remain about how H3K4me3 communicates with microtubules and actin filaments to sustain spindle architecture. The interplay between chromatin modifications and the cytoskeleton represents a cutting-edge frontier with implications for understanding meiotic errors that lead to aneuploidy, a leading cause of developmental disorders. By positioning H3K4me3 at this nexus, the current study underscores the complexity and elegance of oocyte biology.
This research further stresses the significance of epigenetic landscape remodeling during gametogenesis and early development. Unlike somatic cells where gene expression governs much of histone modification dynamics, oocytes at the MII stage utilize these marks to fulfill structural roles vital for genetic stability and developmental potential. Such dual functionalities highlight the versatility of histone modifications and the necessity for nuanced investigation in different biological contexts.
Kyushu University, renowned for its pioneering contributions to reproductive and developmental biology, continues to be at the forefront with this seminal work. Rooted in multidisciplinary expertise and state-of-the-art imaging technologies, the research exemplifies how detailed molecular characterization can illuminate foundational biological processes with far-reaching health implications. The study’s contribution is not merely conceptual but tangibly impacts broader efforts to understand, diagnose, and treat infertility worldwide.
As the global demographic trend leans towards delayed childbearing, the insights into epigenetic regulation of oocyte quality bear even greater urgency. Female reproductive aging presents a formidable challenge, and interventions that maintain chromosomal stability in aging oocytes could revolutionize assisted reproductive technologies (ART). The identification of H3K4me3 as a modulator of chromosomal and spindle stability introduces a promising biomarker and therapeutic target in this landscape.
Finally, this study invites the scientific community to reconsider paradigms surrounding histone modifications, especially their structural and non-transcriptional functions. The multifaceted roles played by H3K4me3 and likely other histone marks in gamete biology urge a re-examination of epigenetic mechanisms in cell division, genome integrity, and developmental competence. The ripple effects of this research will resonate across cell biology, reproductive medicine, and epigenetics, illustrating how detailed molecular discoveries can inform translational advances and clinical innovation.
Subject of Research: Animals
Article Title: Characterization of H3K4me3 in mouse oocytes at the metaphase II stage
News Publication Date: 29-May-2025
Web References: http://dx.doi.org/10.1016/j.jbc.2025.110308
References: Atsushi Takasu, Toshiaki Hino, Osamu Takenouchi, Yasuki Miyagawa, Zhihua Liang, Shota Tanaka, Tomoya Mimura, Chisato Ida, Yuki Matsuo, Yuna Lee, Haruka Ikegami, Miho Ohsugi, Shogo Matoba, Atsuo Ogura, Kazuo Yamagata, Kazuya Matsumoto, Tomoya S Kitajima, Kei Miyamoto, Journal of Biological Chemistry
Image Credits: Kei Miyamoto/Kyushu University
Keywords: H3K4me3, histone modification, metaphase II oocyte, chromosome stability, spindle apparatus, epigenetics, reproductive aging, mouse oocyte, fertility, embryonic development, cytoskeleton, actin cap
Tags: advanced imaging techniques in biologychromosome stabilization mechanismsepigenetic marks in meiosishistone modification H3K4me3Kyushu University research findingsmetaphase II stage significancemolecular manipulation in cell biologymouse oocytes maturationpost-translational modifications in histonesrole of histones in gene regulationspindle architecture in oocytestranscriptionally silent oocytes