Credit: ECDC
In February 2017, a new A(H7N9) virus — indicating high pathogenicity in poultry — was detected in three patients connected to Guangdong, China, as well as in environmental and poultry samples. This is an important development to be monitored, however, ECDC's updated rapid risk assessment concluded that the risk of the disease spreading within Europe via humans is still considered low, as there is no evidence of sustained human-to-human transmission. Although the genetic changes in A(H7N9) may have implications for poultry, to date, there is no evidence of increased transmissibility to humans or sustainable human-to-human transmission.
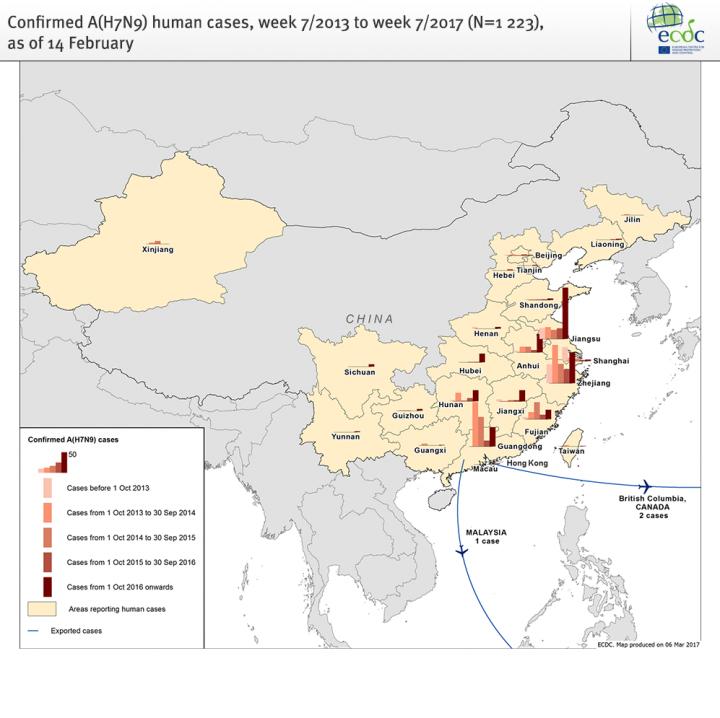
Since the notification of a novel reassortant influenza A(H7N9) virus on 31 March 2013, 1 258 laboratory confirmed cases of human infection with avian influenza A(H7N9) virus have been reported. This is the fifth winter season in the northern hemisphere with human cases caused by A(H7N9) infections. During this wave, the number of human cases has been higher than in previous waves and accounts for 37% of the human cases reported so far. This is most likely due to greater environmental contamination in live bird markets and increased circulation of the virus among poultry.
In February 2017, a new A(H7N9) virus with mutations in the haemagglutinin gene — indicating high pathogenicity in poultry — was detected in two patients from Guangdong and one patient from Taiwan with a travel history to Guangdong, as well as in environmental and poultry samples. However, this new virus has been detected in only three out of 460 human cases confirmed in the current epidemic wave and in one province only. It is unclear at the moment if the newly-emerged, highly-pathogenic avian influenza (HPAI) virus A(H7N9) will replace the low pathogenic virus or if both will co-circulate in the bird population.
Evidence presented at the recent WHO influenza vaccine composition meeting showed both the newly emerged and some of the currently circulating A(H7N9) viruses to be genetically and antigenically distinct from current A(H7N9) candidate vaccine viruses. To address this, new A(H7N9) candidate vaccine viruses were proposed. In addition, the HPAI A(H7N9) strain in these three patients presented with a mutation in the neuraminidase (NA) gene associated with reduced susceptibility to neuraminidase inhibitors. Mutations related to resistance to neuraminidase inhibitors have possibly emerged during the antiviral treatment of the three patients.
The continued transmission of A(H7N9) to humans in China poses the risk that sporadic imported cases may be detected in Europe. The following options for prevention and control of the infection should be considered:
- people travelling to China should avoid direct exposure to poultry and refrain from visiting live poultry markets or backyard farms;
- travellers who have visited affected areas and develop respiratory symptoms and fever upon their return should consult a physician and mention their recent travel history to enable early diagnosis and treatment.
The possibility of humans infected with A(H7N9) returning to the EU/EEA cannot be excluded.
###
Read the rapid risk assessment: Genetic evolution of influenza A(H7N9) virus in China – implications for public health. Sixth update, 9 March
Media Contact
Romit Jain
[email protected]
46-858-601-678
@ECDC_EU
http://ecdc.europa.eu