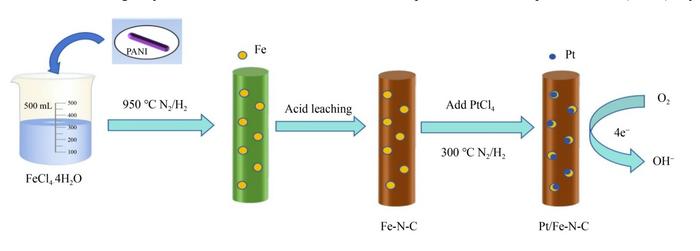
In the rapidly advancing world of sustainable energy technologies, the oxygen reduction reaction (ORR) stands as a critical electrochemical process. It plays a pivotal role in the operation of fuel cells and metal-air batteries, particularly zinc-air systems, which have attracted considerable attention due to their high energy density and environmental friendliness. Despite their promise, a significant bottleneck in mainstream adoption arises from the reliance on platinum (Pt) catalysts, whose prohibitive costs and limited resources act as barriers to scalable commercialization. Addressing these challenges, recent research has unveiled an innovative pathway towards ultra-low platinum loading electrocatalysts, heralding new horizons for high-performance, cost-effective energy conversion devices.
The synthesis mechanism utilizes the inherent advantages of polyaniline chemistry, enabling the synthesis of well-defined Fe-N-C precursors characterized by rich nitrogen coordination environments. These sites uniformly anchor platinum ions during subsequent adsorption steps, encouraging the formation of Pt-Fe bimetallic alloys. Such alloying induces a remarkable electronic interplay between platinum atoms and the Fe-N-C support structure, generating modified electronic states favorable for catalytic turnover. This interaction promotes excellent dispersion of platinum nanoparticles, drastically reducing their size to nanoscale clusters with increased surface area and accessible active sites—features essential for superior catalytic performance.
.adsslot_G7RhQoTABV{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_G7RhQoTABV{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_G7RhQoTABV{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Electrochemical evaluations of the resulting Pt/Fe-N-C catalyst reveal a transformative leap in ORR kinetics. Operating at an ultra-low platinum loading of approximately 1.79 wt%, the catalyst exhibits a doubling of mass activity relative to conventional Pt-based systems. This improvement signifies not just an incremental gain but a paradigm shift, demonstrating that carefully engineered synergistic effects can compensate for—and indeed surpass—traditional platinum demands. Such enhanced intrinsic catalytic activity substantially lowers precious metal usage without trade-offs in performance, which could reshape budget considerations for clean energy technologies.
Beyond activity, catalyst stability is paramount for practical applications. The Pt/Fe-N-C system demonstrates exceptional durability under stringent testing conditions. In alkaline media, the half-wave potential registers a marginal decline of only 20 millivolts even after enduring 10,000 electrochemical cycles, underscoring robust resistance to typical degradation pathways such as nanoparticle agglomeration or detachment. In acidic electrolytes, the catalyst maintains virtually unaltered half-wave potentials over equivalent cycling, an indicator of formidable chemical resilience, crucial for varied fuel cell environments. These findings confirm the capability of the catalyst to sustain high performance during prolonged operational periods.
From a mechanistic perspective, the enhanced ORR activity originates from the intricate synergy between Pt and Fe-N-C catalytic sites. The Fe-N-C matrix not only stabilizes the platinum nanoparticles but also modulates electron density, tuning adsorption energies for oxygen intermediates to favor the reaction pathway. This dual-site cooperative effect facilitates faster reaction kinetics and improves selectivity towards the desirable four-electron reduction process, minimizing undesired peroxide formation and enhancing overall efficiency. Such molecular-level insight underpins the rational design of next-generation catalysts.
This investigation also underscores the critical role of nanoengineering in catalyst design. By controlling not only the composition but also the spatial arrangement and particle size distribution of active sites, the researchers effectively optimize surface chemistry and electronic structure. The polyaniline-mediated fabrication process specifically enables scalable and reproducible production of catalysts with uniformly dispersed bimetallic sites, essential for translating laboratory discoveries into commercial-scale manufacturing.
The reported breakthroughs align with the broader global imperative to decarbonize energy systems sustainably. Fuel cells and metal-air batteries, empowered by cutting-edge catalysts such as Pt/Fe-N-C, are poised to become cornerstones of clean energy infrastructure. The reduction in platinum usage directly addresses cost and supply challenges, opening avenues for the deployment of affordable, high-efficiency fuel cells in transportation, portable electronics, and grid storage applications. Concurrently, improved zinc-air batteries could revolutionize how intermittent renewable energy sources are buffered and delivered with minimal environmental impact.
In summary, the development of an ultra-low platinum loading ORR electrocatalyst integrating platinum with Fe-N-C support exemplifies a significant stride toward next-generation energy conversion technologies. The fusion of elaborate synthetic chemistry, precise nanostructuring, and insightful mechanistic understanding culminates in a catalyst that excels in activity, stability, and cost-effectiveness. As these materials enter further stages of validation and commercialization, they hold promise to transform the sustainable energy landscape, addressing longstanding limitations of precious metal dependency and propelling fuel cell and battery technologies into more widespread use.
This study not only elevates the benchmark for catalyst performance but also charts a strategic framework for future innovations. The approach of leveraging synergistic interactions between noble metals and engineered supports could be extended to other catalytic challenges beyond ORR, including hydrogen evolution and carbon dioxide reduction. Such versatility and scalability will be critical in meeting the diverse demands of a decarbonized global economy, fostering resilient and adaptable energy solutions for decades to come.
Researchers and industry stakeholders alike are now poised to capitalize on these findings, exploring integration avenues within full-cell configurations and pilot-scale deployments. Ongoing efforts will likely focus on refining synthesis protocols, optimizing electrode architectures, and enhancing compatibility with diverse operating conditions. Collectively, these advancements signal a transformative era in electrocatalysis, where materials design, sustainability, and performance converge to realize the full potential of renewable energy technologies.
Subject of Research: Not applicable
Article Title: An ultra-low platinum loading ORR electrocatalyst with high efficiency: Synergistic effects of Pt and Fe-N-C support
News Publication Date: 28-Apr-2025
Web References: 10.1007/s11708-025-1006-4
Image Credits: HIGHER EDUCATION PRESS
Keywords
Energy
Tags: atomic-scale material synthesiscost-effective metal-air batteriesengineered composite electrocatalystsFe-N-C support for catalystshigh-performance fuel cell catalystsnitrogen-doped carbon frameworksoxygen reduction reaction electrocatalystsplatinum substitution with transition metalspolyaniline templating processsustainable energy conversion devicessynergistic interactions in catalysisultra-low platinum loading technology