In a groundbreaking study published in Nature Communications, a team of researchers has unveiled a comprehensive analysis of hereditary haemochromatosis (HH) risk and diagnostic patterns across the British Isles and Ireland. This work marks a significant advancement in understanding the genetic landscape that underpins this common iron overload disorder, shedding light on the nuanced interplay between genotype, phenotype, and population-specific factors that influence disease manifestation. The study not only redefines risk profiles but also proposes refined diagnostic strategies tailored to the genetic diversity observed in this region.
Hereditary haemochromatosis is primarily characterized by excessive intestinal absorption of dietary iron, leading to pathological iron accumulation in multiple organs including the liver, heart, and pancreas. This iron overload can culminate in severe clinical complications such as cirrhosis, cardiomyopathy, diabetes mellitus, and arthropathy, underscoring the need for early identification and management. While mutations in the HFE gene, particularly C282Y and H63D variants, have historically dominated the paradigm of HH genetics, this study expands the scope to include a broader spectrum of loci and haplotypes that modulate risk.
Utilizing an extensive dataset that encompasses whole-genome sequencing and high-resolution genotyping from diverse cohorts resident in the British Isles and Ireland, the researchers delineated the distribution and frequency of pathogenic alleles linked to HH. Their analytical approach integrated advanced population genetics methodologies and bioinformatic pipelines designed to capture both common and rare variants contributing to disease susceptibility. This systemic interrogation revealed a more heterogeneous genetic architecture than previously appreciated, with implications for population-specific penetrance and variable expressivity.
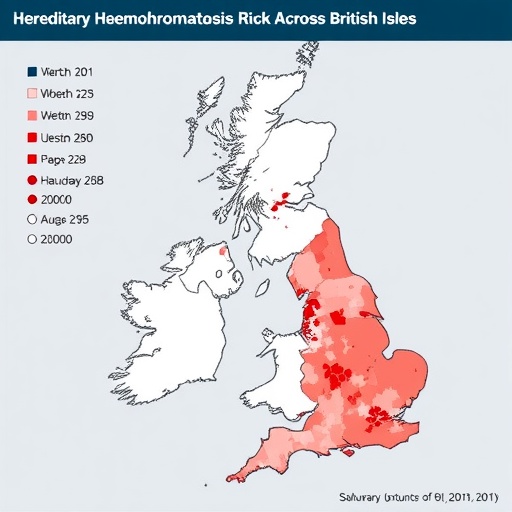
One of the pivotal findings relates to the stratification of HH risk by geographical and ethnic subgroups within the British Isles. The research uncovered marked differences in allele prevalence and risk haplotypes between populations in England, Scotland, Wales, Northern Ireland, and the Republic of Ireland. Such variation correlates with historical migration patterns, founder effects, and genetic drift, illuminating the evolutionary forces that have shaped the current genomic landscape. This granularity underscores the necessity for customized screening protocols that account for local genetic epidemiology.
In addition to classical HFE mutations, the study highlighted the contributory roles of lesser-known variants in genes such as TFR2, SLC40A1, and HAMP, which encode key proteins involved in iron homeostasis. These mutations, although individually rare, aggregate to form a polygenic background that influences iron overload severity and clinical outcomes. The integration of polygenic risk scores (PRS) into diagnostic workflows emerges as a promising avenue to enhance predictive accuracy beyond monogenic testing frameworks.
Clinically, the findings advocate for a paradigm shift in HH diagnosis. The authors argue for the implementation of a tiered genetic screening algorithm that incorporates next-generation sequencing data alongside biochemical markers such as serum ferritin and transferrin saturation. This multiparametric strategy aims to reduce false negatives, capture atypical presentations, and facilitate timely therapeutic interventions such as phlebotomy or chelation therapy to prevent irreversible organ damage.
The study also addresses the challenges posed by population substructure in genome-wide association studies (GWAS) of HH. By applying rigorous correction methods, the researchers minimized confounding and enhanced the resolution of genotype-phenotype correlations. This methodological refinement provides a blueprint for future genetic epidemiology studies seeking to dissect complex traits influenced by both common and rare genetic variations.
Intriguingly, the research uncovers novel haplotype configurations that bear functional significance, implicating regulatory elements modulating gene expression in iron metabolism pathways. Epigenetic landscapes mapped in conjunction with genetic data suggest that non-coding variants in enhancer and promoter regions may subtly alter iron absorption dynamics, offering new mechanistic insights into HH pathophysiology.
Beyond genetics, the study explores environmental and lifestyle factors interacting with genetic predispositions to influence disease penetrance. Variables such as dietary iron intake, alcohol consumption, and metabolic health status are examined in multivariate models, revealing that genetic risk alone does not fully predict disease onset. This highlights the complex gene-environment interdependencies that must be considered in holistic patient management.
The implications of this research extend to public health policies, particularly in the context of population screening and individualized medicine. Tailored genetic counseling informed by detailed risk landscapes can improve patient outcomes through early detection and informed risk mitigation strategies. Moreover, healthcare systems in the British Isles and Ireland may leverage these findings to optimize resource allocation and design prevention programs targeting high-risk groups.
Technologically, the utilization of cutting-edge sequencing platforms and sophisticated bioinformatics tools exemplifies the power of integrative genomics in unraveling complex inherited disorders. This multidisciplinary approach combining clinical genetics, molecular biology, computational science, and epidemiology sets a new standard for investigating multifactorial diseases with variable penetrance.
The authors emphasize the importance of collaborative efforts, incorporating large biobank data and regional clinical registries, to enhance sample diversity and statistical power. Such partnerships enable the identification of subtle risk variants and facilitate validation of diagnostic models across different populations, reinforcing the generalizability and robustness of the findings.
In conclusion, this landmark study redefines the hereditary haemochromatosis landscape by charting the intricate genetic variations and their clinical implications across the British Isles and Ireland. The integration of multi-layered genomic data with clinical phenotypes heralds a new era of precision diagnostics and individualized patient care. As genetic understanding deepens, so does the promise of mitigating the burden of iron overload diseases through early identification, targeted therapies, and informed risk stratification.
This research not only propels the field of haematology forward but also exemplifies the transformative potential of genomics in addressing inherited metabolic disorders. Its comprehensive approach and regional specificity ensure that the findings will resonate with clinicians, researchers, and public health practitioners alike, paving the way for improved outcomes in hereditary haemochromatosis management and beyond.
Subject of Research:
Article Title:
Article References:
Kerr, S.M., Fletcher, B.S., Tzoneva, G. et al. The landscape of hereditary haemochromatosis risk and diagnosis across the British Isles and Ireland. Nat Commun 17, 716 (2026). https://doi.org/10.1038/s41467-025-65511-7
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41467-025-65511-7
Keywords: Hereditary haemochromatosis, HFE gene, iron overload, genetic epidemiology, polygenic risk score, population genetics, British Isles, diagnostics, iron metabolism, genetic screening
Tags: British Isles health studiesdiagnostic strategies for haemochromatosisgenetic analysis of iron overload disordersgenetic diversity and disease riskhereditary diseases in Irelandhereditary haemochromatosis risk factorsHFE gene mutations and variantsiron absorption disorders in adultsiron overload clinical complicationspopulation-specific disease manifestationrefined diagnostic approaches in healthcarewhole-genome sequencing in genetics