In a groundbreaking study recently published in Cell Death Discovery, researchers have unveiled nuanced insights into the therapeutic potential of histone deacetylase inhibitors (HDACi) in addressing autosomal dominant retinitis pigmentosa (adRP), a genetically inherited degenerative eye disorder. This research applied an innovative approach by utilizing the Rho^I255d mouse model, which harbors a specific mutation mirroring conditions in human patients, to dissect the differential effects of HDAC inhibitors on retinal degeneration. The results of this study not only deepen our understanding of adRP’s pathological mechanisms but also open new avenues for targeted pharmacological intervention.
Retinitis pigmentosa (RP) represents a heterogeneous group of inherited retinal dystrophies characterized by progressive photoreceptor cell death, leading invariably to vision loss and, eventually, blindness. The autosomal dominant form accounts for a significant subset of these cases and is often triggered by mutations in the rhodopsin (RHO) gene. The I255d mutation, in particular, has been linked to protein misfolding and toxic aggregation within photoreceptor cells, ultimately impairing visual function. Despite considerable advances in genetic therapies, pharmacological treatment strategies remain limited, with HDAC inhibition emerging as a promising candidate due to its epigenetic regulatory capabilities.
Epigenetic modulators like HDAC inhibitors play an essential role in regulating gene expression by altering chromatin structure, thereby influencing cell survival and death pathways. However, the complexity of epigenetic regulation and its diverse roles in different cell types necessitate a careful examination of HDACi effects, especially in the context of inherited retinal degeneration. The current study tackled this challenge head-on, investigating how specific HDAC inhibitors might differentially modulate retinal health in a controlled animal model replicating adRP pathology.
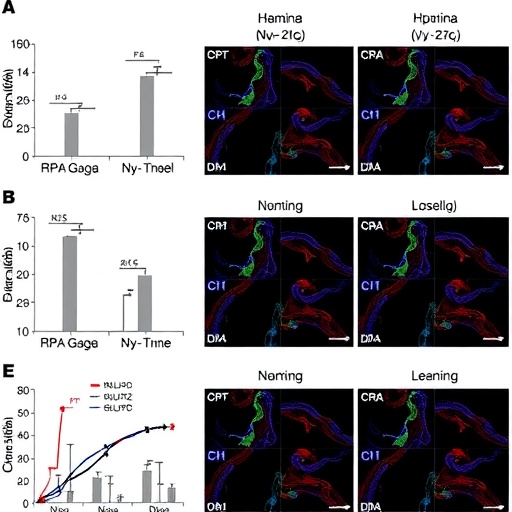
Using the Rho^I255d mouse, the researchers administered various HDAC inhibitors and assessed their distinct impacts on retinal morphology, photoreceptor viability, and molecular signaling pathways. Through a rigorous combination of histological analyses, electrophysiology, and transcriptomic profiling, the team revealed that not all HDAC inhibitors yield the same therapeutic outcomes. Rather, the molecular specificity of these compounds dictates their ability to either alleviate or exacerbate the degenerative process, underscoring the importance of tailored therapeutic design.
One of the most striking findings was the identification of a subset of HDAC inhibitors that effectively preserved photoreceptor cell structure and function in the mutant mice. These agents demonstrated a capacity to attenuate photoreceptor apoptosis and maintain retinal thickness, which translated to measurable improvements in visual function via electroretinography. Conversely, other HDACi compounds exhibited deleterious effects, accelerating photoreceptor loss and exacerbating retinal degeneration, highlighting the perils of indiscriminate application of these epigenetic drugs.
The study meticulously characterized the underlying molecular pathways modulated by the distinct HDAC inhibitors. Protective compounds were shown to upregulate genes involved in cellular stress responses, protein folding, and antioxidative mechanisms, suggesting that these pathways may underlie their beneficial effects. In contrast, inhibitors that worsened retinal outcomes appeared to disrupt vital homeostatic processes and induce pro-apoptotic signaling, emphasizing a delicate balance in epigenetic regulation that must be respected to achieve therapeutic benefit.
Intriguingly, the researchers also uncovered evidence of HDAC isoform-specific effects on retinal cells. Some inhibitors preferentially targeting class I HDACs enabled neuroprotection in the Rho^I255d model, whereas others affecting class II or III HDACs were linked with detrimental consequences. This isoform-selectivity insight provides a critical framework for the future design of more precise HDACi drugs, potentially minimizing off-target effects and maximizing therapeutic efficacy for RP patients.
The implications of these findings extend beyond RP, offering a generalizable model for how tailored epigenetic therapy could be refined to treat diverse neurodegenerative disorders characterized by protein misfolding and cellular stress. By modulating histone acetylation with surgical precision, researchers envisage the development of next-generation therapies that can halt or even reverse neuronal loss in the retina and other vulnerable tissues.
Moreover, this study serves as a clarion call for cautious optimism regarding HDAC inhibitors in retinal therapeutics. While their promise is undeniable, the findings stress that HDAC inhibitors are not a one-size-fits-all solution. The biological complexity inherent in epigenetic interventions demands nuanced understanding and careful clinical translation, especially given the risk of unintended acceleration of disease.
Future research building upon this work might delve into combinatorial approaches that pair isoform-selective HDAC inhibitors with gene therapy or neuroprotective agents to achieve synergistic benefits. Such multilayered strategies could provide durable protection to photoreceptors, slowing the inexorable course of retinal degeneration and preserving vision in affected individuals.
Furthermore, patient-derived induced pluripotent stem cell models, combined with advanced CRISPR technologies, are anticipated to refine this therapeutic paradigm. These cutting-edge tools will allow precise dissection of HDAC function in human retinal tissue contexts, enhancing the translatability of animal model findings to clinical interventions.
This study’s methodological rigor and the depth of molecular insights it offers represent a landmark in adRP research, moving the field closer to actionable therapies for a condition that currently lacks effective treatment. It also highlights the broader therapeutic potential held by epigenetic agents when applied with a mechanistic understanding informed by genetic and biochemical nuance.
In conclusion, the differential effects of HDAC inhibitors presented by Zhu and colleagues revolutionize our conceptual framework for tackling inherited retinal dystrophies. The careful characterization of isoform-specific outcomes in a genetically relevant mouse model lays critical groundwork for personalized medicine approaches that could dramatically improve quality of life for patients battling retinitis pigmentosa and similar neurodegenerative diseases in the years to come.
As we stand on the cusp of an era in which epigenetic therapies transcend from bench to bedside for retinal conditions, studies like this illuminate the path forward. They remind us that the future of vision preservation hinges on precision medicine: therapies designed not only to treat disease but to harmonize with the intricate epigenetic choreography of our cells.
Subject of Research:
The study focuses on the differential effects of histone deacetylase inhibitors on retinal degeneration using the Rho^I255d mouse model of autosomal dominant retinitis pigmentosa.
Article Title:
Differential effects of HDAC inhibitors in the Rho^I255d mouse model for autosomal dominant retinitis pigmentosa.
Article References:
Zhu, Y., Nanda Kumar, P., Jiao, K. et al. Differential effects of HDAC inhibitors in the Rho^I255d mouse model for autosomal dominant retinitis pigmentosa. Cell Death Discov. (2025). https://doi.org/10.1038/s41420-025-02908-9
Image Credits:
AI Generated
DOI:
https://doi.org/10.1038/s41420-025-02908-9
Tags: autosomal dominant retinitis pigmentosa researchgenetic therapies for inherited retinal dystrophiesHDAC inhibitors in retinitis pigmentosahistone deacetylase inhibitors and eye disordersnuanced insights into retinal dystrophiespharmacological interventions for RPprotein misfolding in photoreceptor cellsretinal degeneration and vision lossRhoI255d mouse model studytargeted treatment strategies for retinitis pigmentosatherapeutic potential of epigenetic modulatorsunderstanding adRP pathological mechanisms