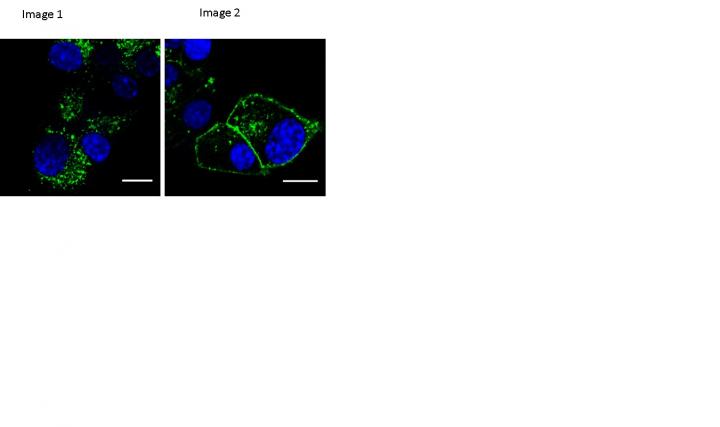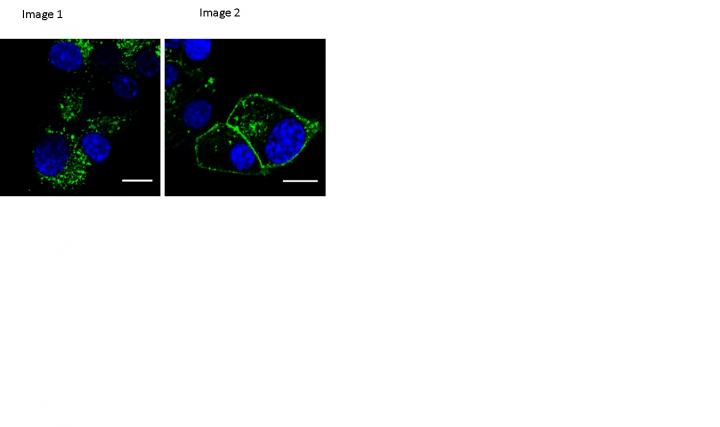
Credit: Imperial College London
Making tiny changes to existing diabetes treatments can alter how they interact with cells, and potentially make the medicines more effective.
The findings come from early-stage studies in both human cells and mice in which researchers tweaked the structure of an existing treatment for type 2 diabetes.
This tweak enabled the researchers, from Imperial College London, to hack into the 'traffic system' that shuttles drugs in and out of cells. This increased the effectiveness of the drug, and led to more insulin being released.
The team, who include a number of international institutions, say a similar approach is already being applied to develop more effective versions of other existing medications, including pain treatments. The technique could also potentially reduce the number of drug side effects.
Patients with type 2 diabetes are unable to effectively control their blood sugar levels. The most commonly prescribed drug is called metformin, but if this doesn't work, or triggers side effects, patients are usually offered other drugs. These include a class of drugs called "incretin mimetics", which stimulate the release of insulin from specialised cells in the pancreas, called beta cells.
In the latest study, researchers focused on one of these compounds, a drug called exenatide, which patients inject twice-daily or weekly, depending on the formulation. The compound acts by binding to and activating a type of docking point – called GLP-1 receptors – found on the surface of beta cells, which stimulate the cells to release insulin.
In a paper, published this week in the journal Nature Communications, researchers explain how switching a few of the building blocks of exenatide could generate new compounds with slightly different properties, changing how they interact with the GLP-1 receptors and making the drugs more effective.
Normally, when GLP-1 receptors are activated – such as when a compound like exenatide binds to them – they move from the cell membrane to the inside of the cell, where they are either degraded, or recycled, i.e. they re-join the cell membrane ready to start the whole process again. This process, called receptor trafficking, can have a big impact on the effect of a drug and the degree of its side effects.
"Under normal conditions, we may not want cells to be continually activated, so these receptors are internalised and no longer accessible to naturally occurring GLP-1 outside of the cell," explained Dr Ben Jones, from the Bloom Lab at Imperial, and first author of the study. "However, when you have a disease where we can get benefit from continual stimulation of receptors, then avoiding this internalisation process could be an advantage.
The team found that one of their compounds, called "exendin-phe1", altered the GLP-1 receptor trafficking process. In trials with human beta cells in the lab they found that their compound reduced the degree to which the receptors left the membrane, leading to more receptors available on the cell surface to bind to the drug.
With the standard treatment, an estimated 90 per cent of GLP-1 receptors would move from the membrane into the cell, with only around 10 per cent being recycled. With the new compound, however, just 30 per cent of the receptors moved into the cell and the majority of those were recycled, returning to the cell membrane.
In mouse studies, the researchers found that while their new compound increased the amount of insulin being secreted from the animals' beta cells in the pancreas, it appeared to have no increased effect on GLP-1 receptors elsewhere in the body – namely GLP-1 receptors in the brain, which are associated with nausea.
"We have harnessed a new mechanism based on receptor trafficking to develop a drug more effective for type 2 diabetes that doesn't appear to carry an increased risk of side effects," explained Dr Jones. "If this treatment were to make it to market, the advantage is that it could be more effective for treating diabetes compared to the existing treatment, and that extra effectiveness would not be accompanied by greater nausea and other side effects."
According to the researchers, tweaking drugs to influence receptor trafficking could lead to improvements for a host of existing treatments. For example, morphine can provide pain relief by targeting opioid receptors in the body, but it can have a number of off-target effects, including constipation and other gastrointestinal effects, as well as depressing the respiratory system.
By changing the structure of opioid drugs to alter receptor "bias" (a phenomenon related to trafficking), researchers have been able to reduce these effects, while maintaining the pain-relieving properties of the drugs.
The Imperial team is now planning a small study with healthy human volunteers to further explore the mechanism of receptor trafficking and how it could be used, which is expected to begin within the coming months.
Dr Alejandra Tomas, a Lecturer in Professor Guy Rutter's Section and co-senior author of the study, said: "We have found that making small changes to drug molecules can dramatically alter receptor trafficking. Ultimately, we're interested in using these compounds as tools to try and understand receptor biology and generate even more effective drugs in the future."
###
'Targeting GLP-1 receptor trafficking to improve agonist efficacy' by Ben Jones, et al. is published in the journal Nature Communications.
Media Contact
Kate Wighton
[email protected]
020-759-42409
@imperialspark
http://www3.imperial.ac.uk/college/news