In the multifaceted realm of cancer research, the pursuit of effective therapeutic strategies remains a critical focus. A recent study led by Sheng, JJ. and colleagues has caught the attention of the scientific community by unveiling groundbreaking insights into the efficacy of GX15-070, particularly in the context of ovarian cancer treatment. This drug not only enhances the effectiveness of niraparib—a well-known inhibitor of poly (ADP-ribose) polymerase (PARP)—but also incites a significant shift in the cellular DNA repair mechanisms involved in combating this challenging malignancy. The findings promise to redefine future therapeutic paradigms for ovarian cancer and potentially for other types of cancers.
At the core of this study is the intricate relationship between DNA repair pathways and cancer cell survival. The research emphasizes the pivotal role that DNA double-strand break repair mechanisms play in cellular responses to DNA damage. Ovarian cancer, characterized by its high rates of genetic mutations and compromised DNA repair pathways, has historically proven to be resistant to standard therapies. Given the importance of DNA repair in maintaining genomic stability, understanding the role of various repair mechanisms can illuminate new treatment strategies.
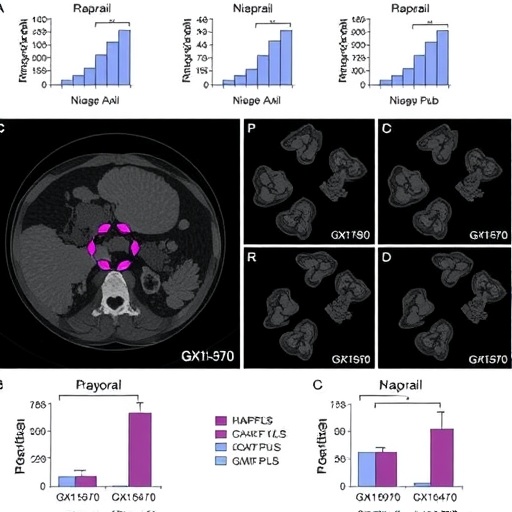
The study meticulously explores the role of Mcl1, a protein critical to cellular survival, in mediating this shift from homologous recombination (HR) to non-homologous end joining (NHEJ)—two primary pathways through which cells repair DNA. In normal physiological conditions, HR is generally favored due to its precision and accuracy in repairing double-strand breaks. However, as the research indicates, GX15-070 facilitates a complex interaction with Mcl1, nudging the repair process towards the less accurate NHEJ pathway. This foundational shift underlines the potential for increased vulnerability in cancer cells, especially when combined with the PARP inhibition provided by niraparib.
Moreover, the implications of this research extend beyond ovarian cancer. The ability to manipulate the DNA repair pathway could revolutionize therapeutic approaches across various malignancies that exhibit similar characteristics. By understanding how to modulate the activity of critical proteins like Mcl1, researchers can explore innovative combination therapies that might enhance the efficacy of existing treatments while minimizing the risk of resistance—an ever-present hurdle in cancer therapy.
As researchers delve deeper into the molecular mechanisms at play, the study offers a treasure trove of data highlighting the precise interactions that underpin these shifts. Detailed analysis revealed that the combined treatment of GX15-070 and niraparib not only improves cell death rates in ovarian cancer models, but also alters gene expression profiles indicative of a shift in repair strategies. Such results provide an invaluable foundation for subsequent clinical trials and could potentially signal a new era in cancer treatment where tailored therapies based on individual tumor profiles could lead to much-needed breakthroughs.
In addition to elucidating these molecular dynamics, the study intricately examines the implications of drug interactions on cellular tolerance and therapeutic resistance. As GX15-070 shifts the balance toward NHEJ, there exists a tangible risk that cancer cells might adapt over time, necessitating rigorous monitoring and the development of additional combination strategies to prevent resistance. These considerations bear great weight on the future landscape of cancer pharmacotherapy, showcasing that innovation must go hand-in-hand with vigilance.
The importance of using clinical models allows researchers to observe these interactions in a more authentic environment, drawing parallels to patient responses. This study thus stands as a beacon of hope, pointing towards a potential pathway whereby more effective treatment regimens can emerge. As researchers strive to bridge bench research with clinical applications, the findings of Sheng et al. underscore the imperative for ongoing collaboration between molecular biologists, oncologists, and pharmacologists to elevate cancer treatment to new heights.
In view of the findings, it is compelling to consider the strategic implications for drug development moving forward. The molecular insights gathered from this study could guide pharmaceutical companies and research institutions in fine-tuning existing drugs or designing novel compounds aimed at enhancing the antitumor effects while concurrently minimizing adverse effects. The dual approach of leveraging both PARP inhibition alongside strategic modulation of DNA repair pathways can herald more lasting therapeutic responses in the complex landscape of cancer.
Building upon these results, further investigations will focus on the safety and efficacy of this combined treatment in diverse populations. Questions remain regarding optimal dosing strategies, the timing of drug administration, and the identification of specific biomarkers that may predict response to such innovative treatment combinations. These avenues of research will be essential to ensure that this emerging therapeutic strategy can be adopted effectively in clinical practices.
The enthusiasm generated by this study reflects a broader trend in oncology toward individualized medicine. The potential to tailor treatments based on a patient’s unique tumor biology presents a transformative shift away from the one-size-fits-all paradigm that has long defined cancer care. Researchers are eager to explore how findings from studies like Sheng et al. can be integrated within ongoing clinical trials that prioritize patient outcomes and quality of life.
In conclusion, the breakthrough findings articulated in this research article motivate an optimistic outlook for future therapies in ovarian cancer and beyond. By elucidating the interplay between GX15-070, niraparib, and Mcl1-mediated pathways, this study forms a cornerstone for future research aimed at combatting the formidable challenges posed by various cancers. As we stand on the precipice of a transformative era in oncology, the integration of molecular insights with clinical strategies has never been more essential.
The future of cancer treatment may very well hinge on similar studies that not only enhance our understanding of tumor biology but also spur innovation in drug development. Embracing the complexity of cancer through comprehensive research will be pivotal in overcoming the limitations of existing therapies and ultimately improving patient outcomes across the globe.
Subject of Research: Ovarian cancer treatment enhancement through modulation of DNA repair pathways.
Article Title: GX15-070 enhances niraparib efficacy in ovarian cancer by promoting a shift in Mcl1-mediated DNA repair pathway from HR to NHEJ.
Article References: Sheng, JJ., He, Y., Liu, PW. et al. GX15-070 enhances niraparib efficacy in ovarian cancer by promoting a shift in Mcl1-mediated DNA repair pathway from HR to NHEJ. J Transl Med 23, 1262 (2025). https://doi.org/10.1186/s12967-025-07284-7
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12967-025-07284-7
Keywords: Ovarian cancer, DNA repair pathways, PARP inhibition, GX15-070, Mcl1, NHEJ, HR.
Tags: cancer treatment resistancecellular responses to DNA damageDNA repair mechanisms in cancergenetic mutations in ovarian cancergroundbreaking cancer research findingsGX15-070 and DNA repair pathwaysGX15-070 ovarian cancer therapyMcl1 protein role in cancer survivalniraparib effectiveness enhancementnovel cancer treatment paradigmsPARP inhibitors in oncologytherapeutic strategies for ovarian cancer