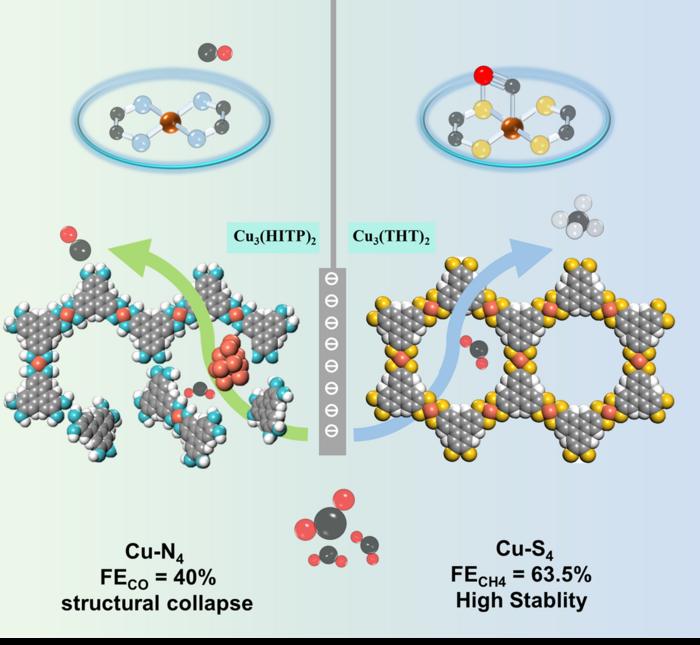
In a remarkable breakthrough in the realm of electrocatalysis, a research team has developed a conductive two-dimensional metal-organic framework (2D MOF), specifically Cu₃(THT)₂, incorporating well-defined copper-sulfur (Cu-S₄) active sites that dramatically enhance the electrochemical reduction of carbon dioxide (CO₂) to methane (CH₄). This study delineates how the presence of sulfur atoms finely modulates the electronic structure of copper centers and establishes additional stabilizing interactions with adsorbed *CO intermediates, thus enabling a highly selective and efficient conversion from CO to the more valuable CH₄. This represents a significant stride in the quest for sustainable and industrially viable CO₂ conversion technologies.
Conventional catalysts often face challenges in tuning product selectivity during CO₂ electroreduction reactions (CO₂RR), commonly favoring the generation of carbon monoxide (CO) or formate at moderate efficiencies. The Cu₃(THT)₂ catalyst, however, achieves an outstanding Faradaic efficiency (FE) of 63.5% toward methane production at a potential of –1.4 V versus the reversible hydrogen electrode (RHE), paired with an industrial-level methane partial current density reaching –189.6 mA cm⁻². Such performance underlines the efficacy of Cu-S₄ coordination environments in stabilizing reaction intermediates leading to C-H bond formations beyond simple CO evolution.
Distinct from Cu₃(THT)₂, the comparative framework Cu₃(HITP)₂—featuring copper-nitrogen (Cu-N₄) sites—exhibits comparatively inferior CO₂RR catalytic behavior. Cu₃(HITP)₂ achieves a CO Faradaic efficiency of merely 40% and undergoes rapid structural decomposition into Cu₂O nanoparticles within just 300 seconds of continuous electrolysis. This stark contrast highlights the pivotal role of sulfur coordination in enhancing both catalytic activity and framework stability under harsh electrochemical conditions.
From a mechanistic perspective, density functional theory (DFT) calculations shed light on the electronic distinctions between Cu-S₄ and Cu-N₄ sites. Sulfur atoms possess lower electronegativity relative to nitrogen, resulting in an increased electron density localized on the Cu centers within the Cu₃(THT)₂ framework. This enhances the overlap with the 5σ and 1π molecular orbitals of the CO intermediate, strengthening σ-donation and π-backbonding interactions critical for CO adsorption and subsequent hydrogenation steps leading to CH₄ formation.
Moreover, the unique properties of the divalent sulfur atoms confer an additional advantage: the capacity to engage in weak yet significant S···O interactions with the oxygen atom of the CO species. These interactions fine-tune the adsorption energy landscape, further optimizing the binding strength of CO. This synergistic effect effectively breaks traditional scaling relationships that have historically limited the selectivity and kinetics of CO₂-to-CH₄ electroreduction on copper-based catalysts.
The robustness of Cu₃(THT)₂ under prolonged electrocatalytic conditions underscores its viability for real-world applications. Continuous operation for over 21,000 seconds shows negligible decline in catalytic activity, an impressive feat in light of typical catalyst degradation issues encountered in CO₂RR systems. The sulfur coordination not only contributes to enhanced electronic properties but also imparts structural integrity, resisting the degradation pathways that plague nitrogen-coordinated analogues.
This study provides a pioneering framework for the inclusion of non-metallic sulfur centers in CO₂ electroreduction catalysts, revealing their critical role in tipping product selectivity from CO—often considered a simple and less valuable output—to methane, which commands higher economic and practical significance as a fuel and chemical feedstock. The approach paves the way for the rational design of next-generation electrocatalysts tailored at the atomic scale to address the global challenge of carbon recycling.
Beyond experimental characterization, theoretical insights confirm that the ligand environment and heteroatom choice within metal-organic frameworks are paramount in dictating reaction pathways and catalyst lifetime. By integrating spectroscopic investigations, computational modeling, and electrochemical analyses, the researchers provide a comprehensive understanding of how electronic and geometric factors intertwine in defining catalytic behavior.
This discovery holds considerable promise for advancing renewable energy technologies and carbon-neutral cycles, as methane produced via electricity-driven CO₂ reduction can seamlessly integrate into existing natural gas infrastructures. The high current density attained by Cu₃(THT)₂ also aligns with industrial scalability requirements, bridging the gap between fundamental catalysis research and practical application.
Looking ahead, the findings spotlight the strategic importance of tailoring local coordination chemistry within conductive MOFs. The delicate balance between electron density, adsorbate binding affinity, and catalyst durability achieved through Cu-S₄ sites could inspire the exploration of other chalcogen-based heteroatoms and framework topologies to optimize the electroreduction of diverse carbon-based substrates.
In essence, the study represents a significant leap forward by demonstrating how non-metallic heteroatoms like sulfur can dramatically alter catalytic landscapes. Such innovation furthers the prospect of deploying designed MOFs as multifunctional platforms not only for CO₂ conversion but also for a wide array of electrocatalytic transformations critical to sustainable chemical synthesis.
The integration of synthetic chemistry, advanced characterization, and state-of-the-art computational methods in this work exemplifies the multidisciplinary approach necessary to unlock the potential of metal-organic frameworks within the energy and environmental sectors. These collective insights are poised to influence future directions in catalyst development aimed at mitigating atmospheric CO₂ levels through economically attractive and industrially viable routes.
This milestone underscores that manipulating the microenvironment of active sites at the molecular level can yield profound effects on product selectivity and catalytic efficiency. As researchers strive toward carbon-neutral energy cycles, such fundamental advances lay the groundwork for unlocking CO₂ as a resource rather than viewing it solely as an environmental liability.
Subject of Research: Electrocatalytic CO₂ reduction using conductive metal-organic frameworks with sulfur-coordinated copper active sites
Article Title: Not specified
News Publication Date: Not specified
Web References: http://dx.doi.org/10.1016/j.scib.2025.01.033
Image Credits: ©Science China Press
Keywords
CO₂ electroreduction, methane production, metal-organic framework, Cu₃(THT)₂, copper-sulfur sites, Faradaic efficiency, catalytic selectivity, electrocatalyst stability, density functional theory, π-backbonding, sulfur coordination, carbon recycling
Tags: carbon dioxide conversion technologiesCO2 electroreductionconductive metal-organic frameworkscopper-sulfur active sitesCu3(THT)2 catalystelectrocatalysis breakthroughsFaradaic efficiency in CO2RRindustrial-level methane generationmethane productionselective CO2 conversionstable reaction intermediatessulfur coordination in catalysts