In recent years, carbon-based catalysts — especially nitrogen-doped nanocarbons — have emerged as sustainable, reliable alternatives to the metal catalysts that have traditionally been used to support chemical reactions. Researchers from the Key Laboratory of Advanced Carbon-Based Functional Materials (Fujian Province University) at Fuzhou University synthesized nanocarbons from guanine molecules to better understand the precise role nitrogen plays in the carbon-based materials and explore the reaction mechanisms of these catalytic systems.
Credit: Zailai Xie; Fuzhou University
In recent years, carbon-based catalysts — especially nitrogen-doped nanocarbons — have emerged as sustainable, reliable alternatives to the metal catalysts that have traditionally been used to support chemical reactions. Researchers from the Key Laboratory of Advanced Carbon-Based Functional Materials (Fujian Province University) at Fuzhou University synthesized nanocarbons from guanine molecules to better understand the precise role nitrogen plays in the carbon-based materials and explore the reaction mechanisms of these catalytic systems.
In a recently published study, the research team clarified how different types of nitrogen can modulate oxidative dehydrogenation activity — a critical process involved in converting inert compounds into reactive nanocarbons.
The study was published in the journal Carbon Future on February 4.
“The study offers theoretical guidance for creating highly effective carbon catalysts, which could advance clean energies converted from renewable resources in industries like plastics, medicine and rubber,” said study author Zailai Xie from Fuzhou University.
Doping carbon materials with heteroatoms such as nitrogen can change the carbon’s properties. This practice has gained significant interest, driving researchers to investigate possible benefits. Nitrogen doping, in particular, has been shown to be a highly effective strategy in creating advanced materials for carbon-dioxide capture, energy conversion, energy storage and other applications.
Despite the strides being taken in the field of nitrogen-doping, there are still some key questions that remain unanswered. For instance, the performance of nanocarbon materials is significantly influenced by functional groups of atoms on the surface — but, so far, nanocarbon materials exhibit uncontrollable surface functional groups, which complicates the identification of active sites for different types of reactions.
“This behavior hinders our understanding of the intrinsic role that nitrogen dopants play in improving catalytic activity and determining the catalytic mechanism,” Xie said.
To further advance the field of nitrogen-doped nanocarbon catalysis, researchers need more controlled and better-characterized catalysts, according to Xie. This would allow researchers to isolate the effects of specific nitrogen species on catalytic performance.
In pursuit of this goal, the Fuzhou University research team developed a method to precisely control surface functional groups, particularly oxygen and nitrogen groups, during nanocarbon catalyst generation.
The team obtained a set of nanocarbons through self-assembling guanine molecules — a compound found in guano or fish scales — and exposed the resulting material to heat in the absence of oxygen. Drawing inspiration from the supramolecular self-assembly of biological components like guanine and related nucleobases such as guanosine, this synthetic approach offers an intriguing means of generating ordered nanomaterials. These molecules possess π-stacked, H-bonded, and other multiplex binding sites that facilitate the formation of functional supramolecular assemblies. Guanine, being widely present in the biogenic photonic structures of various living organisms, exhibits diverse shapes and sizes, including hexagonal plates, square plates, irregular polygons, and prisms. The subtle variations in the morphology of guanine crystals contribute to the colorful optical phenomena observed in animals, such as fish scales, spider bodies, and animal eyes. However, the precise control of the morphology of biogenic guanine crystals in organisms remains poorly understood. Despite the remarkable properties of guanine crystals, the artificial production of regular guanine crystals that closely mimic biological conditions and their subsequent transformation into functional carbon materials has not yet been achieved in chemical synthesis approach.
“The synthesized carbons exhibited unique and intriguing properties, including relatively stable surface oxygen groups and high nitrogen content,” Xie said.
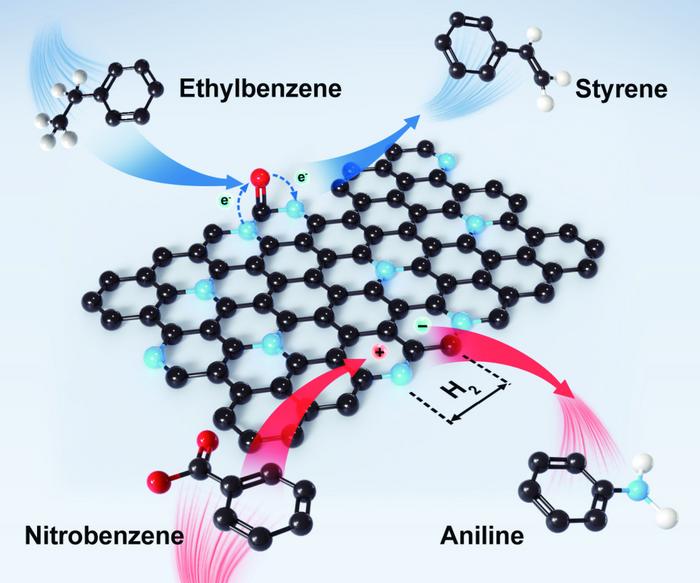
In addition, the presence of multiple hydrogen bonds in guanine enabled the formation of a two-dimensional nanosheet with controllable types of nitrogen dopants. The nitrogen content can be finely tuned from approximately 5% to 30 at%, while the oxygen content can be kept at a consistent 4%.
“This unique property makes guanine an ideal proof-of-concept precursor for construction model catalysts that can lead to in-depth understanding of the role of high nitrogen dopants in nanocarbon catalysis,” Xie said.
To further probe the structure-function relationships, the team tested with dehydrogenation and hydrogenation reactions, in which hydrogen molecules are stripped off or added to a larger molecule. The tests demonstrated that different types of nitrogen in the nanocarbons, namely graphitic nitrogen and pyridinic nitrogen, serve as electron-donating and electron-withdrawing modulators, respectively, which can tailor the oxidative dehydrogenation activity of the nanocarbons.
“As an efficient, metal-free catalyst, we have unraveled the roles of nitrogen dopants in both dehydrogenation and hydrogenation for the first time,” said Xie. “We believe that our findings provide valuable insights into the physical-chemical reaction mechanisms of nitrogen-doped carbon catalytic systems and offer theoretical guidance for the synthesis of highly effective carbon catalysts.”
The research is supported by the National Natural Science Foundation of China and the Natural Science Foundation of Fujian Province.
Other contributors include Xinying Lin, Xiaoyan Huang, Yiquan Chen, Sen Lin, Xing Huang and Xuefei Zhang from Fuzhou University.
About Carbon Future
Carbon Future is an open access, peer-reviewed and international interdisciplinary journal that reports carbon-related materials and processes, including catalysis, energy conversion and storage, as well as low carbon emission process and engineering. Carbon Future will publish Research Articles, Reviews, Minireviews, Highlights, Perspectives, and News and Views from all aspects concerned with carbon. Carbon Future will publish articles that focus on, but not limited to, the following areas: carbon-related or -derived materials, carbon-related catalysis and fundamentals, low carbon-related energy conversion and storage, low carbon emission chemical processes.
About SciOpen
SciOpen is a professional open access resource for discovery of scientific and technical content published by the Tsinghua University Press and its publishing partners, providing the scholarly publishing community with innovative technology and market-leading capabilities. SciOpen provides end-to-end services across manuscript submission, peer review, content hosting, analytics, and identity management and expert advice to ensure each journal’s development by offering a range of options across all functions as Journal Layout, Production Services, Editorial Services, Marketing and Promotions, Online Functionality, etc. By digitalizing the publishing process, SciOpen widens the reach, deepens the impact, and accelerates the exchange of ideas.
Journal
Carbon Future
DOI
10.26599/CF.2024.9200008
Article Title
Identification of role of nitrogen dopants in nanocarbon catalysis
Article Publication Date
4-Feb-2024




