Embryonic stem (ES) cells are pluripotent stem cells that can produce all cell types of an organism. ES cells proliferate rapidly and have been thought to experience high levels of intrinsic replication stress. However, a recent report published in EMBO Reports by Kurashima et al. challenges this assumption by providing a detailed molecular investigation of replication dynamics in these cells.
Embryonic stem (ES) cells are pluripotent stem cells that can produce all cell types of an organism. ES cells proliferate rapidly and have been thought to experience high levels of intrinsic replication stress. However, a recent report published in EMBO Reports by Kurashima et al. challenges this assumption by providing a detailed molecular investigation of replication dynamics in these cells.
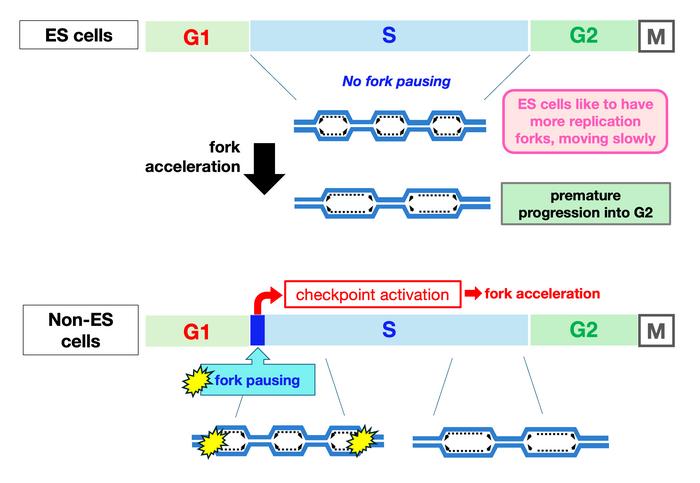
Led by Dr. Tomomi Tsubouchi at the National Institute for Basic Biology (NIBB) in Japan, the research team explored replication fork dynamics – such as replication fork speed, pausing frequency, and origin firing density – across different substages of the S phase. Their findings reveal previously overlooked aspects of replication not only in ES cells but also in other non-pluripotent cells.
Dr. Kiminori Kurashima, the first author in the study, explains, “By fractionating cells in substages of the S phase and performing DNA fiber assays on these sorted populations, we discovered that mammalian pluripotent stem cells maintain a slow fork speed and high active origin density throughout the S phase, with minimal fork pausing”. This contrasts with their finding that in non-pluripotent cells, which exhibit slower fork speeds at the beginning of the S phase but accelerate thereafter. Non-pluripotent cells also experience fork pausing specifically in the early S phase, likely activating checkpoint mechanisms to facilitate fork acceleration and reduce pausing.
The research also shows that upon differentiation, mouse ES cells adopt replication characteristics similar to those of non-pluripotent cells. Furthermore, some features of DNA replication observed in mouse ES cells are shared with human iPS cells, suggesting that the slow replication fork with high origin density may be a hallmark of pluripotency. Surprisingly, forcing an acceleration of replication forks led to miscoordination between genome replication completion and cell cycle progression.
Dr. Tsubouchi concludes, “We propose that slow replication forks are not the manifestation of replication impediments but rather an integral feature of DNA replication in ES cells. Our study underscores the dynamic regulation of DNA replication and highlights how different cell types employ distinct mechanisms.”
Journal
EMBO Reports
DOI
10.1038/s44319-024-00207-5
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Embryonic stem cells maintain high origin activity and slow forks to coordinate replication with cell cycle progression
Article Publication Date
25-Jul-2024