Credit: Lawrence Berkeley National Laboratory
We see crystals all around us: snowflakes, ice cubes, table salt, gemstones, to name a few. Invisible to the naked eye, but of special interest to scientists, are crystalline “nanowires” — wires with a diameter of a mere few nanometers and a typical length of a micrometer.
Normally in a rod-like shape, these wires are an interesting area of worldwide research because of their many potential applications, including semiconductors and miniaturized optical and optoelectronic devices.
As reported in a recent Nature paper, scientists at the Center for Nanoscale Materials (CNM), a U.S. Department of Energy (DOE) Office of Science User Facility located at Argonne National Laboratory, played a critical role in the discovery of a DNA-like twisted crystal structure created with a germanium sulfide nanowire, also known as a “van der Waals material.” The research was conducted in collaboration with the University of California at Berkeley and Lawrence Berkeley National Laboratory.
“It is amazing that these inorganic germanium sulfide nanowires so closely resemble the organic DNA structure,” said co-author Jianguo Wen, a CNM materials scientist. “Nature creates remarkable structures beyond our imagination.”
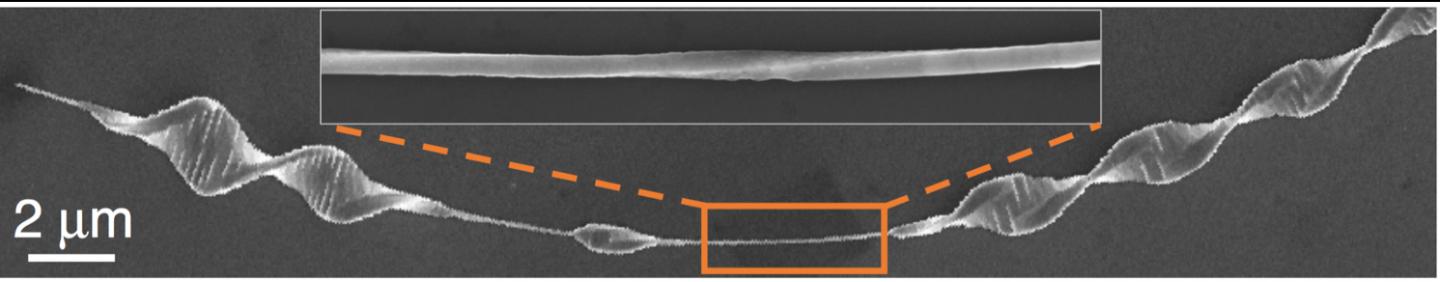
The helical DNA-like structure forms spontaneously by giving the nanowire an “Eshelby twist.” Co-first author Jie Wang, a former materials scientist in CNM (now at Thorlabs, Inc.), explained that the term “Eshelby twist” refers to its discoverer, John Eshelby.
While a research associate working at the University of Illinois at Urbana-Champaign in the 1950s, Eshelby conducted an important theoretical analysis of “screw dislocation” in a thin rod. Relating the effect to crystals, Wang noted that the “screw dislocation occurs when stress is applied to a rod shape in which the atoms become rearranged in a helical pattern.”
When applied to a germanium sulfide nanowire, this twisting causes it to elongate and widen into a helical structure.
“It is amazing that these inorganic germanium sulfide nanowires so closely resemble the organic DNA structure,” said co-author Jianguo Wen, a CNM materials scientist. “Nature creates remarkable structures beyond our imagination.”
Also important, added CNM scientist and co-author Dafei Jin, was the finding that the nanostructure automatically divides into segments that resemble helically stacked bricks. These brick-like segments arise from the release of energy as the wire diameter grows from tens of nanometers to micrometers.
“The discovered Eshelby twist here offers a new way to engineer nanomaterials,” said Wang. “We can tailor these nanowires in many different ways — twist periods from two to twenty micrometers, lengths up to hundreds of micrometers, and radial dimensions from several hundred nanometers to about ten micrometers.”
By this means, researchers can adjust the electrical and optical properties of the nanowires to optimize performance for different applications.
“This is an important materials discovery,” Wen said. “We are excited to have figured out, using CNM’s high-resolution transmission electron microscope, the dislocation structures that drive the nanowires to have an Eshelby twist.”
###
The researchers’ work described above appears in the June 20 issue of Nature and is entitled “Helical van der Waals crystals with discretized Eshelby twist.” Authors are Yin Liu, Jie Wang, Su Jung Kim, Haoye Sun, Fuyi Yang, Zixuan Fang, Nobumichi Tamura, Ruopeng Zhang, Xiaohui Song, Jianguo Wen, Bo Z. Xu, Michael Wang, Shuren Lin, Qin Yu, Kyle B. Tom, Yang Deng, John Turner, Emory Chan, Dafei Jin, Robert O. Ritchie, Andrew M. Minor, Daryl C. Chrzan, Mary C. Scott, and Jie Yao.
This work was supported by the Office of Basic Energy Sciences, U.S. Department of Energy, and performed, in part, at Argonne’s Center for Nanoscale Materials.
About Argonne’s Center for Nanoscale Materials
The Center for Nanoscale Materials is one of the five DOE Nanoscale Science Research Centers, premier national user facilities for interdisciplinary research at the nanoscale supported by the DOE Office of Science. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE’s Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge, Sandia and Los Alamos National Laboratories. For more information about the DOE NSRCs, please visit https:/
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https:/
Media Contact
Diana Anderson
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




