German doctors on Friday gave details of how an experimental drug together with advanced intensive care helped save a Ugandan physician who had been airlifted from Sierra Leone with Ebola.
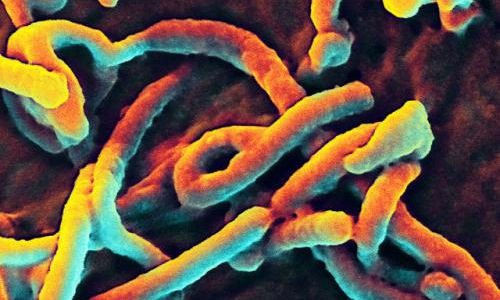
A scanning electron micrograph of Ebola virus budding from a cell (African green monkey kidney epithelial cell line). Photo Credit: NIAID
A prototype drug called FX06, designed to stop haemorrhage, was given to the patient after the doctors got special authorisation from their hospital’s ethics committee, they reported in The Lancet.
“Even though the patient was critically ill, we were able to support him long enough for his body to start antibody production and for the virus to be cleared by his body’s defences,” said Timo Wolf of University Hospital Frankfurt.
The Frankfurt team and the makers of the experimental drug had announced its use in early November.
Publication in The Lancet, a leading peer-reviewed medical journal, validates this announcement.
The unnamed 38-year-old male doctor had been airlifted to Frankfurt in early October, five days after the onset of Ebola symptoms, and admitted to a Biosafety 4 facility, the highest level of medical security, the study said.
Within three days of admission, he was suffering from failure of the lungs, kidneys and gastro-intestinal tract, as well as haemorrhaging blood vessels, a hallmark of Ebola infection.
He was placed on a ventilator and kidney dialysis and administered with antibiotics and a three-day course of FX06.
Called a fibrin-derived peptide, FX06 is designed to seal off the walls of blood vessels, which become permeable when infected by a haemorrhagic virus.
The peptide works by binding to the surface of endothelial cells, which form the inner cell layer of blood vessels. It latches onto the cells via a target called VE-cadherin.
The drug was invented at Vienna General Hospital and is made by a small Austrian firm called MChE-F4Pharma.
It had previously been tested on lab mice infected with the dengue virus and was also put through a trial among 234 European patients to assess its potential for limiting damage to cardiac tissue after a heart attack.
The combination of intensive care and the drug helped the Ugandan patient to stabilise and then recover, the doctors reported.
After a 30-day observation period, no trace of Ebola was found in his blood, and he was released from hospital to return to his family.
But the Lancet study also reported that shortly after this case, another Ebola patient with acute Ebola and haemorrhage was treated with FX06 at a hospital in the eastern Germany city of Leipzig, but died.
Treating Ebola cases in intensive care is a task with “complexity and specific challenges,” it warned.
FX06 is “a potentially valuable therapeutic candidate” for fighting the disease, the authors said, calling for the drug to be assessed in clinical trials.
More than 6,900 people have died in the latest Ebola epidemic, which is centred on the west African states of Guinea, Liberia and Sierra Leone, the World Health Organization (WHO) said on Wednesday.
Story Source:
The above story is based on materials provided by The Lancet.