Researchers in the RIKEN Center for Brain Science (CBS) examined the genetics of autism spectrum disorder (ASD) by analyzing mutations in the genomes of individuals and their families. They discovered that a special kind of genetic mutation works differently from typical mutations in how it contributes to the condition. In essence, because of the three-dimensional structure of the genome, mutations are able to affect neighboring genes that are linked to ASD, thus explaining why ASD can occur even without direct mutations to ASD-related genes. This study appeared in the scientific journal Cell Genomics on January 26.
Credit: Riken
Researchers in the RIKEN Center for Brain Science (CBS) examined the genetics of autism spectrum disorder (ASD) by analyzing mutations in the genomes of individuals and their families. They discovered that a special kind of genetic mutation works differently from typical mutations in how it contributes to the condition. In essence, because of the three-dimensional structure of the genome, mutations are able to affect neighboring genes that are linked to ASD, thus explaining why ASD can occur even without direct mutations to ASD-related genes. This study appeared in the scientific journal Cell Genomics on January 26.
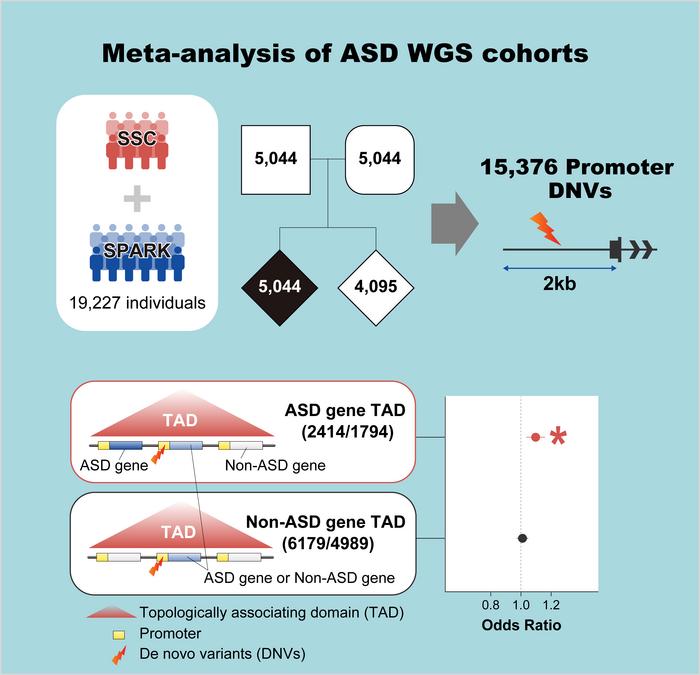
ASD is a group of conditions characterized in part by repetitive behaviors and difficulties in social interaction. Although it runs in families, the genetics of its heritability are complex and remain only partially understood. Studies have shown that the high degree of heritability cannot be explained simply by looking at the part of the genome that codes for proteins. Rather, the answer could lie in the non-coding regions of the genome, particularly in promoters, the parts of the genome that ultimately control whether or not the proteins are actually produced. The team led by Atsushi Takata at RIKEN CBS examined “de novo” gene variants—new mutations that are not inherited from one’s parents—in these parts of the genome.
The researchers analyzed an extensive dataset of over 5,000 families, making this one of the world’s largest genome-wide studies of ASD to date. They focused on TADs—three-dimensional structures in the genome that allow interactions between different nearby genes and their regulatory elements. They found that de novo mutations in promoters heightened the risk of ASD only when the promoters were located in TADs that contained ASD-related genes. Because they are nearby and in the same TAD, these de novo mutations can affect the expression of ASD-related genes. In this way, the new study explains why mutations can increase the risk of ASD even when they aren’t located in protein-coding regions or in the promotors that directly control the expression of ASD-related genes.
“Our most important discovery was that de novo mutations in promoter regions of TADs containing known ASD genes are associated with ASD risk, and this is likely mediated through interactions in the three-dimensional structure of the genome,” says Takata.
To confirm this, the researchers edited the DNA of stem cells using the CRISPR/Cas9 system, making mutations in specific promoters. As expected, they observed that a single genetic change in a promotor caused alterations in an ASD-associated gene within the same TAD. Because numerous genes linked to ASD and neurodevelopment were also affected in the mutant stem cells, Takata likens the process to a genomic “butterfly effect” in which a single mutation dysregulates disease-associated genes that are scattered in distant regions of the genome.
Takata believes that this finding has implications for the development of new diagnostic and therapeutic strategies. “At the very least, when assessing an individual’s risk for ASD, we now know that we need to look beyond ASD-related genes when doing genetic risk assessment, and focus on whole TADs that contain ASD-related genes,” explains Takata. “Further, an intervention that corrects aberrant promoter-enhancer interactions caused by a promotor mutation may also have therapeutic effects on ASD.”
Further research involving more families and patients is crucial for better understanding ASD’s genetic roots. “By expanding our research, we will gain a better understanding of the genetic architecture and biology of ASD, leading to clinical management that enhances the well-being of affected individuals, their families, and society,” says Takata.
Journal
Cell Genomics
DOI
10.1016/j.xgen.2024.100488
Method of Research
Experimental study
Subject of Research
People
Article Title
Topologically associating domains define the impact of de novo promoter variants on autism spectrum disorder risk
Article Publication Date
26-Jan-2024




