Credit: KAIST
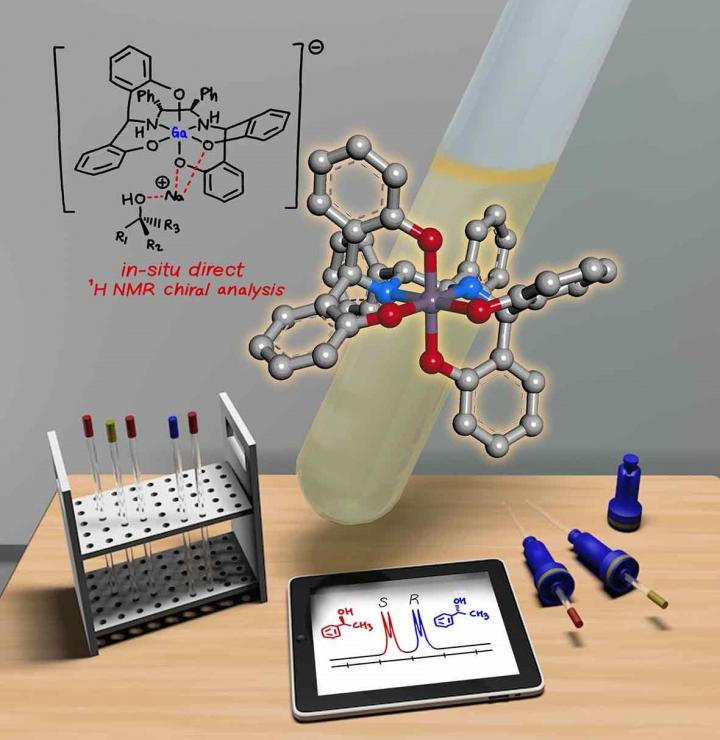
A KAIST research team has developed a gallium-based metal complex enabling the rapid chiral analysis of alcohols. A team working under Professor Hyunwoo Kim reported the efficient new alcohol analysis method using nuclear magnetic resonance (NMR) spectroscopy in iScience.
Enantiopure chiral alcohols are ubiquitous in nature and widely utilized as pharmaceuticals. This importance of chirality in synthetic and medicinal chemistry has advanced the search for rapid and facile methods to determine the enantiomeric purities of compounds. To date, chiral analysis has been performed using high-performance liquid chromatography (HPLC) with chiral columns.
Along with the HPLC technique, chiral analysis using NMR spectroscopy has gained tremendous attention as an alternative to traditionally employed chromatographic methods due to its simplicity and rapid detection for real-time measurement. However, this method carries drawbacks such as line-broadening, narrow substrate scope, and poor resolution. Thus, compared with popular methods of chromatographic analysis, NMR spectroscopy is infrequently used for chiral analysis.
In principle, a chiral solvating agent is additionally required for the NMR measurement of chiral alcohols to obtain two distinct signals. However, NMR analysis of chiral alcohols has been challenging due to weak binding interactions with chiral solvating agents. To overcome the intrinsic difficulty of relatively weak molecular interactions that are common for alcohols, many researchers have used multifunctional alcohols to enhance interactions with solvating agents.
Instead, the KAIST team successfully varied the physical properties of metal complexes to induce stronger interactions with alcohols rather than the strategy of using multifunctional analytes, in the hopes of developing a universal chiral solvating agent for alcohols. Compared to the current method of chiral analysis used in the pharmaceutical industry, alcohols that do not possess chromophores can also be directly analyzed with the gallium complexes.
Professor Kim said that this method could be a complementary chiral analysis technique at the industry level in the near future. He added that since the developed gallium complex can determine enantiomeric excess within minutes, it can be further utilized to monitor asymmetric synthesis. This feature will benefit a large number of researchers in the organic chemistry community, as well as the pharmaceutical industry.
###
Profile:
Professor Hyunwoo Kim
Department of Chemistry
KAIST
http://mdos.
[email protected] ???
About KAIST
KAIST is the first and top science and technology university in Korea. KAIST was established in 1971 by the Korean government to educate scientists and engineers committed to industrialization and economic growth in Korea.
Since then, KAIST and its 61,125 graduates have been the gateway to advanced science and technology, innovation, and entrepreneurship. KAIST has emerged as one of the most innovative universities with more than 12,000 students enrolled in five colleges and seven schools including 1,000 international students from 80 countries.
On the precipice of its 50th anniversary in 2021, KAIST continues to strive to make the world better through the pursuit in education, research, entrepreneurship, and globalization.
Media Contact
Younghye Cho
[email protected]
82-423-502-294
Original Source
https:/
Related Journal Article
http://dx.




