Perovskites are among the most extensively studied materials in modern materials science. Their often unique and exotic properties, which stem from perovskite’s peculiar crystal structure, could find revolutionary applications in various cutting-edge fields. One intriguing way of realizing such properties is through the precise ordering of a perovskite’s defects, such as vacancies or substitutions.
Credit: Tokyo Tech
Perovskites are among the most extensively studied materials in modern materials science. Their often unique and exotic properties, which stem from perovskite’s peculiar crystal structure, could find revolutionary applications in various cutting-edge fields. One intriguing way of realizing such properties is through the precise ordering of a perovskite’s defects, such as vacancies or substitutions.
In oxide chemistry, scientists have known for a long time that oxide defects can spontaneously and consistently arrange themselves throughout the crystal lattice, once they reach certain concentrations (e.g. integer ratio). This emerging order can give rise to attractive properties. While defect ordering has been observed numerous times in perovskite oxides, the same cannot be said about hybrid halide perovskites, composed of an organic cation, a metal cation, and a halide anion.
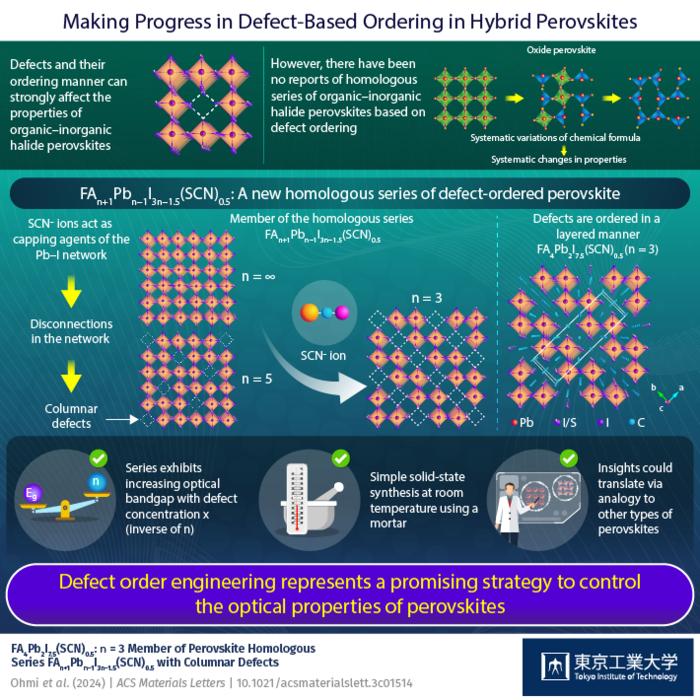
Interestingly, in a recent study published in ACS Materials Letters, a research team including Associate Professor Takafumi Yamamoto from Tokyo Institute of Technology discovered a new defect-ordered layered halide perovskite, shedding light on how order can emerge through defects in these compounds. This work was inspired by a previous finding reported by the researchers, namely the formation of ‘defect columns’ obtained by introducing thiocyanate ion (SCN−) into the crystal lattice of FAPbI3 to obtain FA6Pb4I13.5(SCN)0.5. “We hypothesized that, if the concentration of SCN in the lattice increased, the amount of the PbI columnar defects would also increase, leading to different types of defect ordering, as seen in vacancy-ordered perovskite oxides,” explains Dr. Yamamoto.
The team synthesized FAPbI3 perovskite powders and single crystals via solid-state reactions using precisely defined concentrations of starting materials, including specific ratios of SCN–. They found that when an appropriately high ratio of SCN– was used, the obtained perovskite was represented by the formula FA4Pb2I7.5(SCN)0.5. This layered compound, like the previously reported one, also exhibited columnar defects spanning all stacked layers. However, unlike FA6Pb4I13.5(SCN)0.5, in which one-fifth of the PbI columns were orderly defected, one-third of all columns in the new FA4Pb2I7.5(SCN)0.5 were defects.
The main novelty of this discovery is that the new compound, alongside the previous one, forms what’s known as a ‘homologous series.’ This means that systematic variations of the compound’s chemical formula, which can be represented using integer variables, result in systematic changes in its properties. In this case, the researchers found that the optical bandgap of the material increased with the concentration of ordered defects in the lattice.
Worth noting, this work presents the first homologous series based on defect ordering found for hybrid organic–inorganic perovskites. “This study provides a new playground for defect engineering in organic–inorganic hybrid perovskite compounds. We believe that this new field has the potential to develop by an analogy to the defect-ordering already seen in perovskite oxides,” remarks Dr. Yamamoto. “We have also provided a new strategy to control the defect orderings for tuning a perovskite’s optical properties by incorporating SCN–.”
With any luck, these findings will translate to progress in an exciting area of materials science, ultimately leading to new perovskites with useful qualities for next-generation technologies.
Journal
ACS Materials Letters
DOI
10.1021/acsmaterialslett.3c01514
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
FA4Pb2I7.5(SCN)0.5: n = 3 Member of Perovskite Homologous Series FAn+1Pbn−1I3n−1.5(SCN)0.5 with Columnar Defects
Article Publication Date
17-Apr-2024




