Messenger RNA (mRNA) technology has become popular in the last few years due to its use in COVID-19 vaccines. This technology has been so groundbreaking that it recently won the 2023 Nobel Prize in medicine “for discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.” This isn’t new technology, however— modified mRNAs have been studied for decades and show significant potential for therapeutic applications. Compared to unmodified mRNAs, modified mRNAs are more stable and have more favorable immunogenic effects.
Credit: The Grainger College of Engineering at the University of Illinois Urbana-Champaign
Messenger RNA (mRNA) technology has become popular in the last few years due to its use in COVID-19 vaccines. This technology has been so groundbreaking that it recently won the 2023 Nobel Prize in medicine “for discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.” This isn’t new technology, however— modified mRNAs have been studied for decades and show significant potential for therapeutic applications. Compared to unmodified mRNAs, modified mRNAs are more stable and have more favorable immunogenic effects.
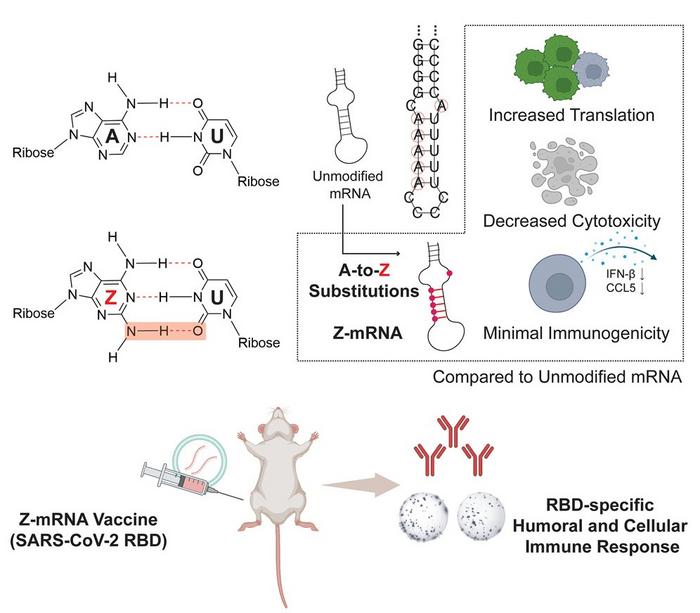
A team of researchers from the University of Illinois Urbana-Champaign have incorporated a newly discovered base, called base Z (2-aminoadenine), into mRNA to create Z-mRNA that has improved translational capacity, decreased cytotoxicity and drastically reduced immunogenicity compared to unmodified mRNA.
The results from this new research, led by chemical and biomolecular engineering professor Huimin Zhao, former chemical and biomolecular engineering graduate student Meng Zhang (current postdoc at Stanford University), and postdoc Nilmani Singh (Carl R. Woese Institute for Genomic Biology), were recently published in the journal iScience.
“We incorporated base Z into mRNA, and we wondered whether it would perform better than the current modified mRNA vaccine platform,” says Zhao.
mRNA is a macromolecule that is transcribed from DNA and contains the instructions for protein production in cells. For use in vaccines, the mRNA is used to encode the antigen that triggers an immune response in human bodies. This way, the body is “trained” to fight off certain viruses and if infected with the real thing, the body can recognize it and produce the necessary antibodies.
Unmodified mRNA contains four bases—A, G, C and U. In this research, base A was replaced with base Z, which can form three hydrogen bonds, a weak bond between two molecules from an attraction of positive and negative charges, with base U (unlike two hydrogen bonds formed between typical A:U pairing). Moreover, base Z differs from other modified bases because it does not come from the human body. “Traditional wisdom is that modified bases should ideally come from the human body so that any mRNA modified by such a base would mimic mRNA in the human body and bypass immune surveillance,” Zhang says.
Despite not naturally existing in the human body, base Z mRNA still demonstrated low immunogenicity—the ability of cells to provoke an immune response and generally considered to be an undesirable physiological response—and reduced cytotoxicity (causing cell death) when tested in cultured cells. While mRNA-based vaccines in general induce lower immune responses, many people still experience some sort of immune reaction. “Having an mRNA vaccine alternative with less side effects would be a really big deal,” says Singh.
To demonstrate the application of their Z-mRNA in vivo, the team developed a Z-mRNA-based COVID-19 vaccine. They tested this Z-mRNA vaccine, alongside the modified mRNA vaccine used by Moderna and Pfizer, in mice, and they found that the Z-mRNA vaccine could induce a substantial and antigen-specific immune response. Although the potency of the Z-mRNA vaccine was not as strong as the current standard, the team believes Z-mRNA could be further improved through systematic engineering efforts.
“The biggest implication of this work is that people don’t have to be limited by those modifications that are naturally present in our body, it is possible to choose modifications outside of our body, but that can still give the desired biological effects such as reduced immunogenicity and enhanced translational ability,” says Zhang.
“We proved that our new modified mRNA vaccine has reduced cytotoxicity and also minimal immunogenicity, which has been a problem in the past for mRNA-based therapies,” says Zhao. “mRNA-based therapeutics are a hot area currently. Not just for vaccine applications, but even for cancer treatments. We want to take advantage of the unique features of Z-containing mRNA for other therapeutic applications.”
*
Huimin Zhao is also an affiliate of the Department of Chemistry, the Department of Biochemistry, and the Department of Bioengineering at UIUC.
Other contributors to this work include Mary Elisabeth Ehmann (Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign) and Lining Zheng (Department of Materials Science and Engineering at the University of Illinois at Urbana-Champaign).
This research was funded by the Steve L. Miller Endowed Chair fund and partly by the U.S. National Institutes of Health.
https://doi.org/10.1016/j.isci.2023.107739
Journal
iScience
DOI
10.1016/j.isci.2023.107739
Article Title
Incorporation of noncanonical base Z yields modified mRNA with minimal immunogenicity and improved translational capacity in mammalian cells
Article Publication Date
26-Aug-2023




