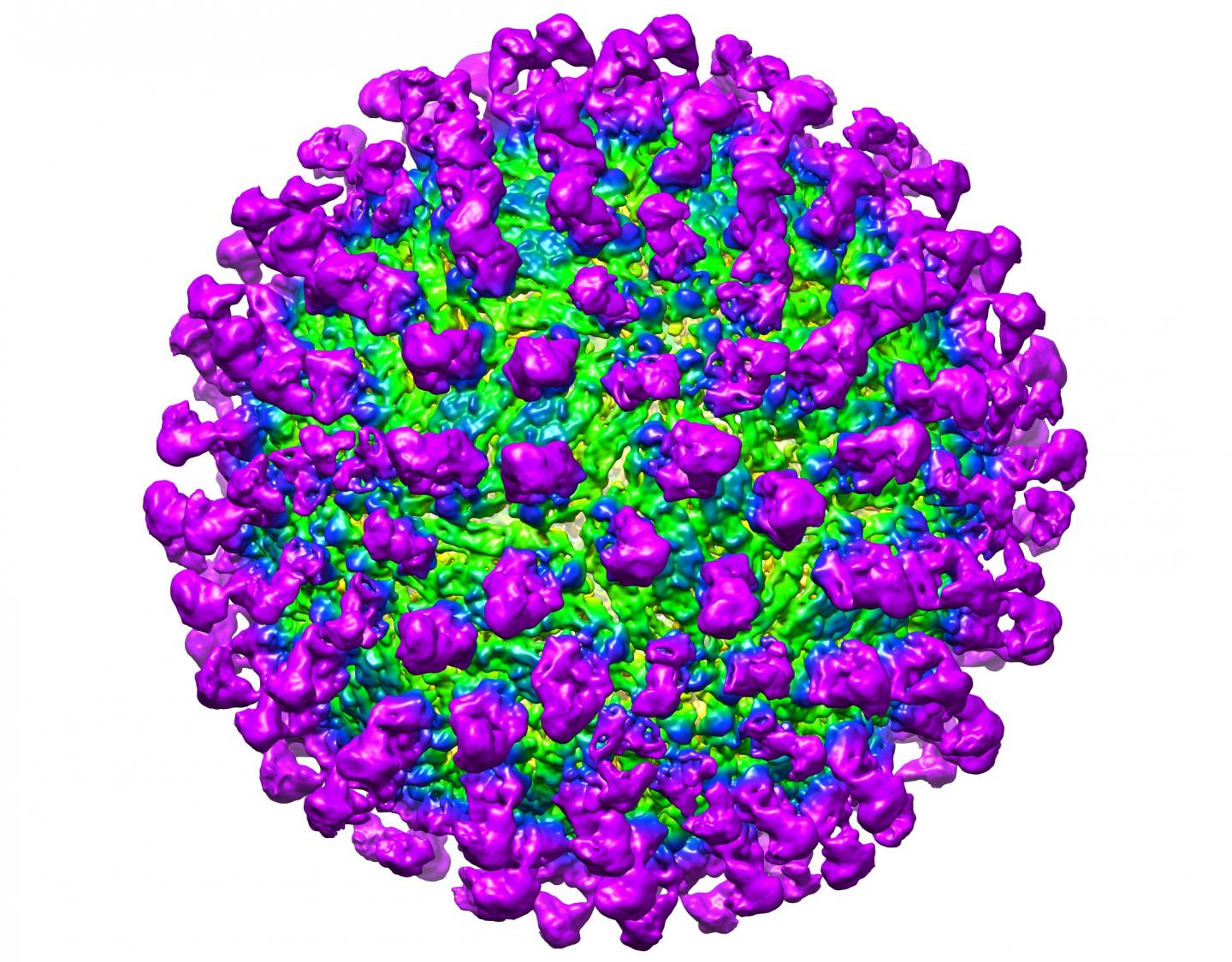
Credit: Victor Kostyuchenko, Duke-NUS Medical School
As Zika spreads throughout the world, the call for rapid development of therapeutics to treat Zika rings loud and clear. Taking a step further in identifying a possible therapeutic candidate, a team of researchers at Duke-NUS Medical School (Duke-NUS), in collaboration with scientists from the University of North Carolina, have discovered the mechanism by which C10, a human antibody previously identified to react with the Dengue virus, prevents Zika infection at a cellular level.
Previously, C10 was identified as one of the most potent antibodies able to neutralise Zika infection. Now, Associate Prof Lok Shee-Mei and her team at the Emerging Infectious Disease Programme of Duke-NUS have taken it one step further by determining how C10 is able to prevent Zika infection.
To infect a cell, virus particles usually undergo two main steps, docking and fusion, which are also common targets for disruption when developing viral therapeutics. During docking, the virus particle identifies specific sites on the cell and binds to them. With Zika infection, docking then initiates the cell to take the virus in via an endosome – a separate compartment within the cell body. Proteins within the virus coat undergo structural changes to fuse with the membrane of the endosome, thereby releasing the virus genome into the cell, and completing the fusion step of infection.
Using a method called cryoelectron microscopy, which allows for the visualisation of extremely small particles and their interactions, the team visualised C10 interacting with the Zika virus under different pHs, so as to mimic the different environments both the antibody and virus will find themselves in throughout infection. They showed that C10 binds to the main protein that makes up the Zika virus coat, regardless of pH, and locks these proteins into place, preventing the structural changes required for the fusion step of infection. Without fusion of the virus to the endosome, viral DNA is prevented from entering the cell, and infection is thwarted.
"Hopefully, these results will further accelerate the development of C10 as a Zika therapy to combat its effects of microcephaly and Guillain-Barré syndrome. This should emphasise the need for further studies of the effect of C10 on Zika infection in animal models," commented Dr Lok.
"By defining the structural basis for neutralization, these studies provide further support for the idea that this antibody will protect against Zika infection, potentially leading to a new therapy to treat this dreaded disease," says Ralph Baric, PhD, professor in the Department of Epidemiology at UNC's Gillings School of Global Public Health.
These findings suggest that C10 may be developed as a therapy for Zika infection, and should be further explored. In addition, disrupting fusion with C10 may prove to be more effective in preventing Zika infection compared with therapies that attempt to disrupt docking. This is because the fusion step is critical for Zika infection, while the virus may develop other mechanisms to overcome disruptions to the docking step. With the call for rapid development of Zika therapies, C10 has emerged as a front runner to answer this call.
Published on 24 November 2016 in Nature Communications, this research is supported by the Singapore Ministry of Education Tier 3 Grant (MOE2012-T3-1-008), the National Research Foundation Investigatorship Award (NRF-NRFI2016-01 to Lok Shee-Mei), Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore, and National Institute of Health (USA) AID Research Grants (AI100625, AI107731 to Ralph S. Baric).
###
Media Contact
Yen May Ong
[email protected]
65-984-11321
@dukenus
http://www.duke-nus.edu.sg
############
Story Source: Materials provided by Scienmag