Credit: ©Science China Press
Solar-driven reduction of CO2 into energy-rich fuels, such as CO, HCOOH, and CH3OH, has been conceived as a highly promising approach to solve energy crisis and environmental pollution. Throughout the molecular photocatalytic systems, numerous catalysts, such as complexes of Re, Ru, Fe, Co and Ni, have been developed with detail study of their catalytic mechanism. In light of their relative mature study, more and more attention has begun to shift to accelerate electron transfer between catalyst and antenna molecules to promote CO2 reduction. At present, the research in this field focuses on the formation of composite systems between photosensitizers and catalysts through chemical bonds, hydrogen bonds, etc. This system shortens the distance between photosensitizers and catalysts, thus improving the electron transport capability between them. However, these studies still have many disadvantages, such as lack of flexibility and great influence from external factors. Accordingly, it’s highly necessary yet remains great challenging to develop alternative strategy for dramatically boosting photocatalytic CO2 reduction.
At present, improving photosensitization ability of PSs for enhancing photocatalytic performance for CO2 reduction is still in its infancy. In this field, the frequently used PSs are confined to prototypical MLCT (metal-to-ligand charge transfer) complexes, such as Ru(bpy)32+ and Ru(phen)32+ (Phen = 1,10-phenanthroline), where their excited state lifetime was usually less than 1 μs (τ= 600 ns for Ru(bpy)32+ and 360 ns for Ru(phen)32+ in CH3CN). It will be a promising way to boost CO2 reduction via adjusting excited state population and lifetime of these PSs to improve their sensitizing ability.
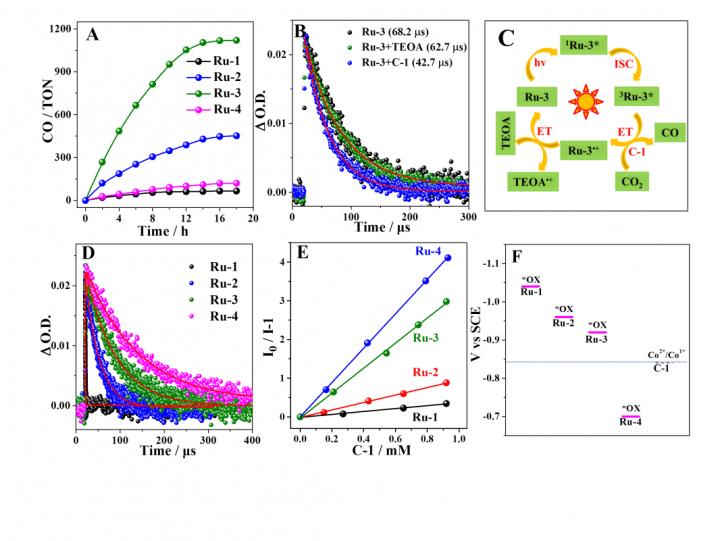
In the present work, researchers put forward a new strategy to greatly boost photocatalytic CO2 reduction by improving photosensitization ability of PSs. A family of Ru(II)-based PSs Ru-2, Ru-3, and Ru-4 were prepared by selective addition of pyrene / pyrenyl ethynylene to 3- and 5-positions of Phen in Ru(Phen)32+ (Ru-1) (Fig. 1). As the triplet state energy level gradually decreased from Ru-1 with 3MLCT state to Ru-4 with 3IL state, the triplet lifetimes of these complexes were gradually prolonged and their excited state oxidation potentials became less negative, providing a platform to compare the effect of PSs with different sensitizing ability on photocatalytic CO2 reduction.
The photocatalytic process was dominated by oxidation mechanism for Ru-1 – Ru-4-containing system (Fig. 2). From the view of kinetics, long-lived triplet state of PSs greatly contributed to intermolecular electron transfer / energy transfer. Thus stern-volmer quenching constants of PSs by C-1 were in the order of 4.4 × 103 M-1 for Ru-4 > 3.2 × 103 M-1 for Ru-3 > 9.6 × 102 M-1 for Ru-2 > 3.8 × 102 M-1 for Ru-1, which was proportional to their excited state lifetimes (Fig. 2D). From the thermodynamics viewpoint, excited state oxidation potentials of PSs determine the driven force of electron transfer from excited PSs to C-1. As shown in Fig. 2F, the absolute value of excited state oxidation potential was in the order of Ru-4
This work provides a new insight for dramatically boosting photocatalytic CO2 reduction via improving photosensitization.
###
See the article:
Improving Photosensitization for Photochemical CO2-to-CO Conversion.
Ping Wang, Ru Dong, Song Guo,* Jianzhang Zhao, Zhi-Ming Zhang,* and Tong-Bu Lu
https:/
Media Contact
Zhi-Ming Zhang
[email protected]
Related Journal Article
http://dx.




