Credit: Brian Foelsch, Geisinger Health System
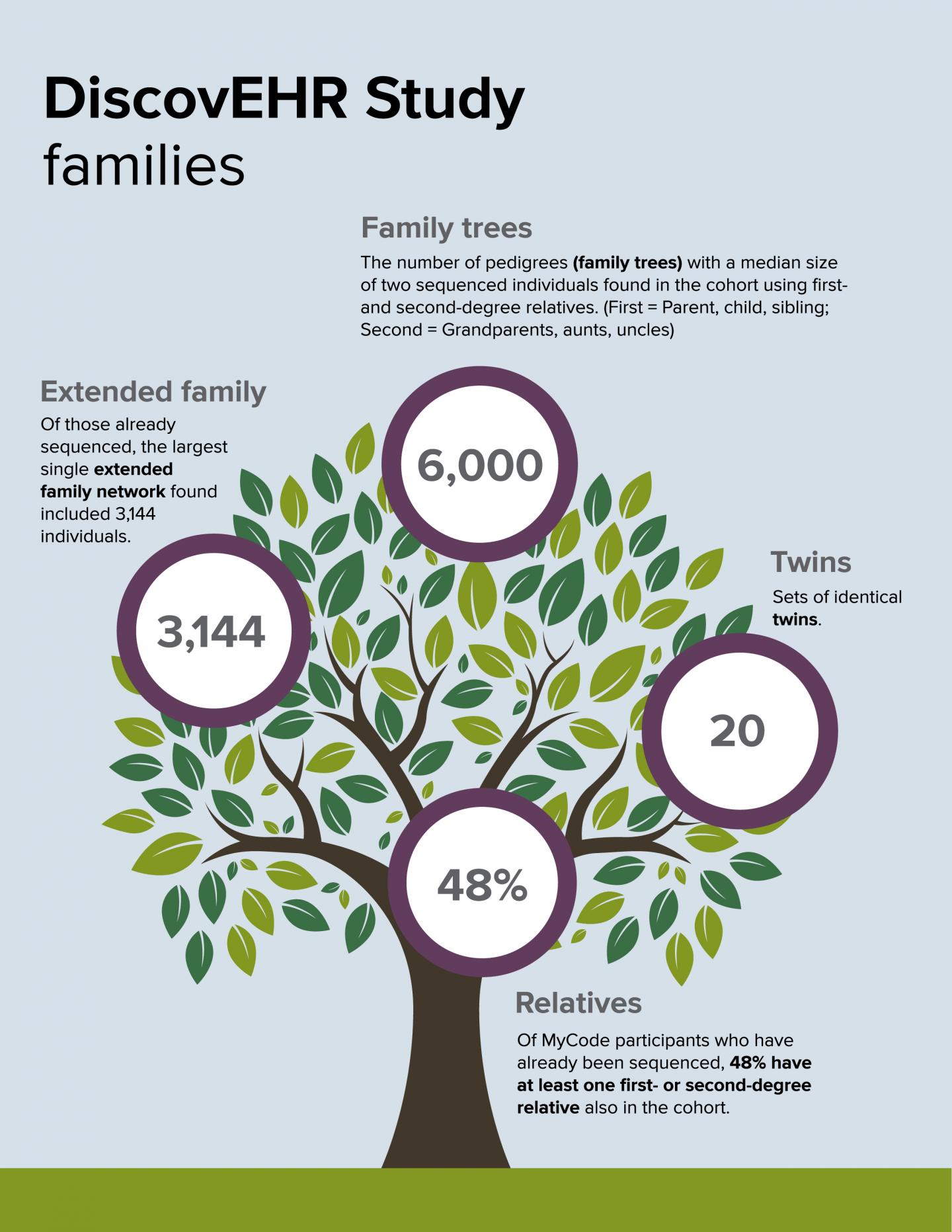
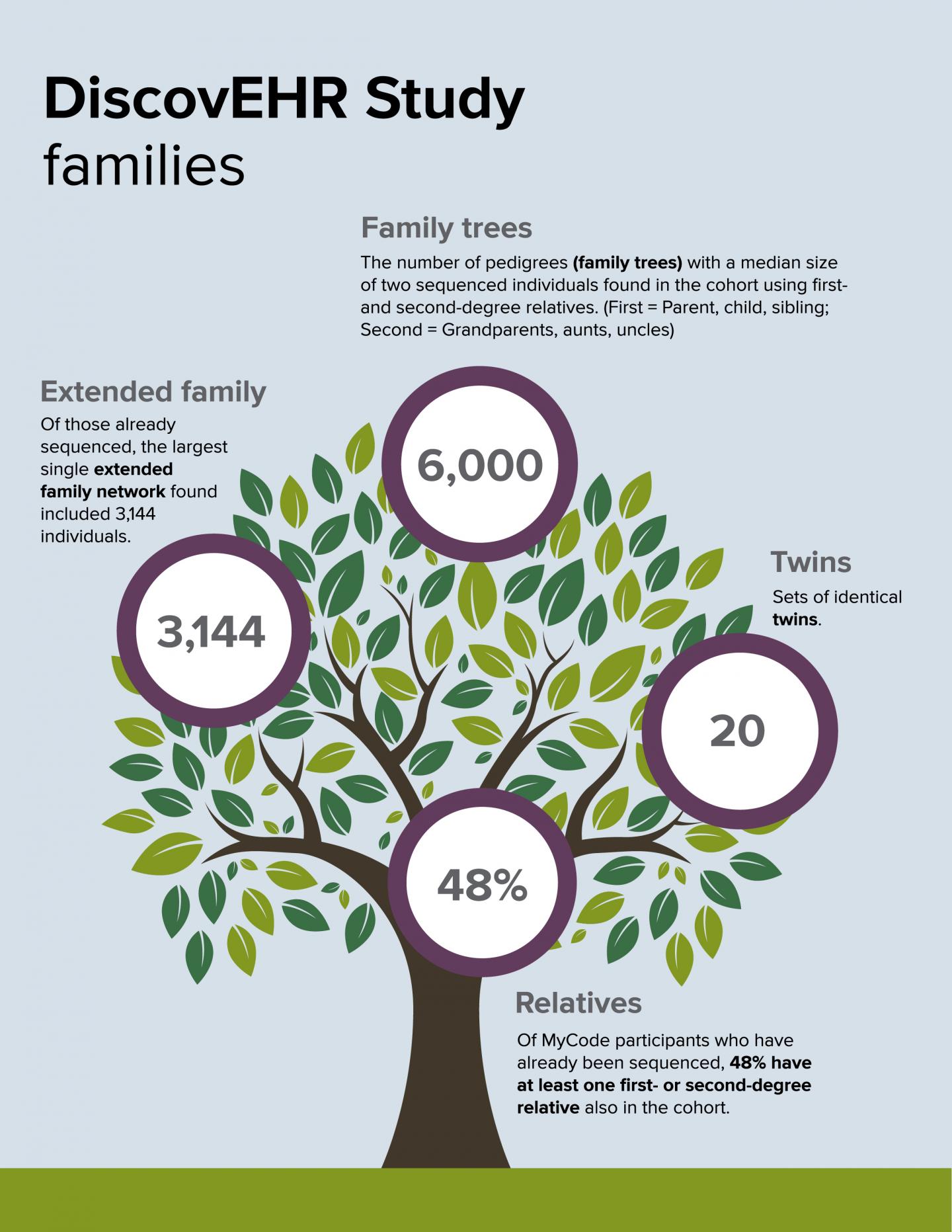
DANVILLE, Pa. – A study conducted by Geisinger Health System in collaboration with the Regeneron Genetics Center (RGC) has found that a life-threatening genetic disorder known as Familial Hypercholesterolemia (FH) is both underdiagnosed and undertreated. It was published in the peer-reviewed journal Science on Dec. 23 alongside another significant study from the same Geisinger-RGC collaboration known as DiscovEHR. That other study describes exome sequencing and analyses of the first 50,726 adult participants in the DiscovEHR cohort – all members of the Geisinger MyCode Community Health Initiative.
In the FH study, the collaborators examined genetic variants causing FH in the DiscovEHR cohort and then compared the findings against the de-identified medical histories of these patients as contained in Geisinger electronic health records. Traditionally, in the United States, FH is diagnosed in patients with high cholesterol who also have a family history of early heart attacks and strokes. Genetic testing for FH is currently uncommon in clinical practice.
FH is caused by a defect that makes the body unable to remove "bad" cholesterol from the blood. This cholesterol (low density lipoprotein cholesterol or LDL-C) then accumulates, often undetected, and can lead to early death from heart attacks or stroke – even in very young people.
Results of the new study found many undiagnosed cases of FH and helped to define the extent of FH in the general population.
"The study shows us that FH is about twice as common as it was once thought to be, and that large-scale genetic testing for FH helps identify cases that would otherwise be missed," said Michael F. Murray, M.D., Geisinger director of clinical genomics. "We now hope to use DNA sequencing to guide better management for patients."
Among the many findings of the study were that 1 in every 256 people has a disease-causing mutation, or variant, in one of the three FH genes. It showed that participants with a deleterious FH gene variant had significantly higher "bad" cholesterol than those without an FH gene variant. They also had significantly increased odds of both general and premature coronary artery disease.
"Being able to connect patient's de-identified medical records with their DNA data is an advantage that few others in this field have. Paired with the RGC's unique technological and analytical resources, we are able to make meaningful discoveries that may advance the implementation of precision medicine today and the development of new or improved medicines tomorrow," said Noura Abul-Husn, M.D., Ph.D., associate director of translational genetics at the RGC and co-author of the paper.
The study identified 35 mutations, or variants, in the genes LDLR, APOB, and PCSK9 that have been determined to cause FH. Only 24 percent of people who carry FH-causing variants had sufficient criteria within their electronic health records to support a probable or definite FH diagnosis, meaning that without genetic confirmation, many of these patients would go undiagnosed and likely undertreated. Indeed, 42 percent of people with these FH-causing variants did not have a recent active prescription for statins, the first line therapy for cholesterol lowering. Among statin-treated people with FH-causing variants, less than half met goals for cholesterol lowering.
"Geisinger is committed to translating this important research directly into improved care for our patients," said Geisinger Executive Vice President and Chief Scientific Officer David H. Ledbetter, Ph.D. "We have begun a major effort to confirm individual patient findings and inform individual participants and their doctors when genetic findings, that are known to cause illness, are discovered in our population," he said.
FH is one of 27 genetic conditions being targeted at Geisinger. So far, nearly 200 patients – including 29 FH carriers – have already been informed they carry one or more disease-causing genetic mutations with consequences that can be treated. These conditions are mainly related to risk for cancer or cardiovascular illness. The effort to return individual results will continue as more findings are confirmed. For details see go.geisinger.org/results.
###
About MyCode
The MyCode Community Health Initiative is a precision medicine project, originally launched in 2006, at Geisinger Health System in which Geisinger patient-participants have consented to donate blood and other biological samples to a system-wide biobank designed to store those samples for wide research uses and for genomic or precision medicine.
About DiscovEHR
The DiscovEHR human genetics study population for this analysis includes 50,726 adult Geisinger Health System patients who agreed to participate in the MyCode Community Health Initiative. More than 125,000 MyCode participants have consented into the program to date. MyCode volunteers have given informed consent to allow sharing of de-identified electronic health records, provide samples that can be linked to their health records for broad research, and permit re-contact for additional studies. Electronic health records for this group are available for a median of 14 years of clinical care.
For patients who have been sequenced as part of DiscovEHR, Geisinger will confirm preliminary research findings in a lab facility that is certified to the Clinical Laboratory Improvement Amendments (CLIA) Act, the federal standard for clinical testing. Qualified Geisinger personnel will provide the results of confirmatory CLIA-certified testing to patients and their primary care providers along with genetic counseling and appropriate referrals to other specialists.
About Geisinger
Geisinger Health System is an integrated health services organization widely recognized for its innovative use of the electronic health record and the development of innovative care delivery models such as ProvenHealth Navigator® and ProvenCare®. As one of the nation's largest health service organizations, Geisinger serves more than 3 million residents throughout 45 counties in central, south-central and northeast Pennsylvania, and also in southern New Jersey with the addition of AtlantiCare, a National Malcolm Baldrige Award recipient. The physician-led system is comprised of approximately 30,000 employees, including nearly 1,600 employed physicians, 12 hospital campuses, two research centers and a 551,000-member health plan, all of which leverage an estimated $10.5 billion positive impact on the Pennsylvania and New Jersey economy. Geisinger has repeatedly garnered national accolades for integration, quality and service. In addition to fulfilling its patient care mission, Geisinger has a long-standing commitment to medical education, research and community service. For more information, visit http://www.geisinger.org, or follow the latest Geisinger news and more on Twitter and Facebook.
Media Contact
David Stellfox
[email protected]
717-810-9673
http://www.geisinger.edu
############
Story Source: Materials provided by Scienmag