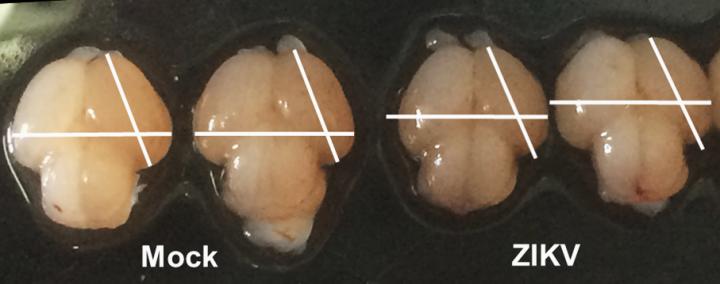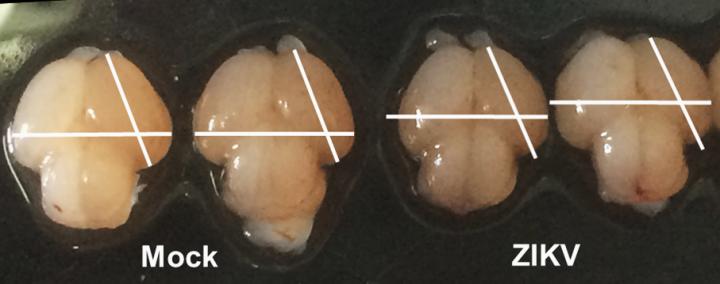
Credit: Li, Xu, Ye, and Hong et al./Cell Stem Cell 2016
Mouse fetuses injected with the Asian Zika virus strain and carried to term within their pregnant mothers display the characteristic features of microcephaly, researchers in China report May 11 in Cell Stem Cell. As expected, the virus infected the neural progenitor cells, and infected brains reveal expression of genes related to viral entry, altered immune response, and cell death. The authors say this is direct evidence that Zika infection causes microcephaly in a mammalian animal model.
The research was a collaborative effort between Zhiheng Xu at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences and Cheng-Feng Qin at the Beijing Institute of Microbiology and Epidemiology.
"The most surprising part of this study is that it was mostly neural progenitor cells that got infected in the beginning and mostly neurons that became infected at a later stage–5 days after injection when the presence of Zika virus increases several hundred folds." says co-senior author Xu. "However, almost all cell death was found in neurons other than neural progenitor cells. This indicates that neurons, but not neural progenitor cells, are prone to induced cell death by the Zika virus."
Zika virus was injected directly into fetal mouse brains. If given too early, the embryos didn't survive, so the researchers began by looking at the equivalent of the second trimester in humans, when the fetus's neural progenitor cells are intensively expanding and generating new neurons at the same time. With this model, they could observe as the brain shrunk with the increase in viral load combined with the intense immune response.
The mice survived to birth but were eaten by their mothers, which often occurs if pups are noticeably unwell, preventing further observation. To overcome this problem, the researchers plan to use lower doses of the Zika virus to see whether that will affect survival. The researchers are also working to identify potential drugs that could reverse the process of Zika-virus-induced microcephaly in mice.
"Mice are not humans, and we need be careful when translating our findings into human disease," says Qin, the other co-senior author on the paper. "Extensive experimental and clinical investigations are urgently needed in response to this global crisis."
"Our animal model, together with the global transcriptome datasets of infected brains, will provide valuable resources for further investigation of the underlying cellular and molecular mechanisms and management of Zika virus-related pathological effects during neural development," Xu says.
###
This work was supported in part by grants from the NSF (China) and MOST (China) "973" program.
Cell Stem Cell, Li, Xu, Ye, and Hong et al.: "Zika virus disrupts neural progenitor development and leads to microcephaly in mice" http://www.cell.com/cell-stem-cell/fulltext/S1934-5909(16)30084-4
Cell Press Statement on Data Sharing in Public Health Emergencies
The Cell Press family of journals is committed to ensuring that the global response to public health emergencies is informed by the best available research evidence and data, and as such, we will make all content concerning the Zika virus free to access. We will work in partnership with reviewers to fast-track review all submissions concerning Zika. We will adapt the editorial criteria that we apply to Zika submissions by asking reviewers to evaluate only if the research methods are sound and support the conclusions and if the work will contribute in some way toward resolving the immediate challenges. We will expedite publication of papers that meet these two criteria.
Cell Stem Cell (@CellStemCell), published by Cell Press, is a monthly journal that publishes research reports describing novel results of unusual significance in all areas of stem cell research. Each issue also contains a wide variety of review and analysis articles covering topics relevant to stem cell research ranging from basic biological advances to ethical, policy, and funding issues. Visit: http://www.cell.com/cell-stem-cell. To receive Cell Press media alerts, contact [email protected]
Media Contact
Joseph Caputo
[email protected]
617-397-2802
@CellPressNews
http://www.cellpress.com