Researchers from the University of Tsukuba identify the protein thrombospondin-1 as a novel extracellular mediator of mechanotransduction triggered by mechanical stress
Credit: University of Tsukuba
Tsukuba, Japan – Cells and tissues are far from being mere static structures. They have the ability to sense and dynamically react to external cues to ensure that they adapt to the ever-changing outside environment. Now, researchers from the University of Tsukuba have identified a novel protein that plays a central role in the transduction of external mechanical cues to cells in blood vessel walls. In a new study, they show how the protein thrombospondin-1 (Thbs1) ensures that blood vessel walls are strengthened during times of mechanical stress and conversely, how the absence of Thbs1 can result in weakened blood vessel walls.
All tissues, including blood vessels, are composed of different types of cells and proteins surrounding them, the latter also being called extracellular matrix (ECM). The ECM not only ensures that cells are stably anchored to an outside structure, but also serves as a means to transmit mechanical impulses. In blood vessels, the most important mechanical cues stem from the pulsatile blood flow as well as the constantly changing blood pressure. While a large number of proteins have been described to play a role in the interplay between cells and the ECM, a clear understanding of how this mechanical microenvironment is coordinated to maintain the structural and functional integrity of blood vessels has been lacking.
“Blood vessels are constantly exposed to strong mechanical forces from the outside, so they have to adapt to them by translating these cues into proper cellular responses,” says corresponding author Professor Hiromi Yanagisawa. “The goal of our study was to further our understanding of the interaction between blood vessels and mechanical stress during normal biology and during times of high stress, such as in hypertension.”
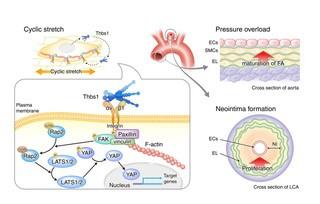
To achieve their goal, the researchers exposed rat vascular smooth muscle cells (SMCs) to cyclic stretch to mimic pulsatile blood flow and found that mechanical stress resulted in SMCs producing more Thbs1 and secreting it. They then discovered that Thbs1 interacted with certain integrins, a protein family that links cells to the ECM, to strengthen so called focal adhesions, which are anchor points that help maintain cellular tension as well as correctly orient cells in response to mechanical stretch.
“These findings show how Thbs1 plays a significant role in the cellular response to relatively mild mechanical stress,” says lead author Professor Yoshito Yamashiro. “We next wanted to know how Thbs1 is involved in more extreme cases of mechanical stress, such as during severe hypertension.”
To simulate hypertension, the researchers performed transverse aortic constriction (TAC) in mice. TAC forces the heart to pump blood against a severely narrowed aorta, resulting in high blood pressure and high mechanical stress on the aortic wall. Although all normal mice survived this procedure, one third of mice lacking Thbs1 died of aortic rupture as a result of weakened aortic wall due to disrupted interaction between blood vessel wall cells and the ECM.
“These are striking results that show how Thbs1 is a central extracellular mediator of mechanotransduction,” says Professor Yanagisawa. “Our findings provide new insights into the biomechanics of the vessel wall, which is relevant to hypertension, one of the most prevalent diseases today. We hope that our findings will facilitate the development of novel therapeutic options for cardiovascular diseases.”
###
The article, “Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP signaling pathway in the remodeling of blood vessels,” was published in Proc. Natl. Acad. Sci. USA
Media Contact
Naoko Yamashina
[email protected]




