Credit: AlphaMed Press
Durham, NC – A study released today in STEM CELLS Translational Medicine suggests a new way to correct facial atrophy of localized scleroderma (LoS) in patients. It shows how applying grafts made up of the patient’s own fat enhanced with adipose?derived stem cells (ADSCs) is a safe, feasible and attractive alternative to conventional fat grafting or fat grafting combined with stromal vascular fraction in treating this condition.
LoS is a rare autoimmune disease caused when the body makes too much collagen, which results in the skin becoming stiff and hard. “Presenting mainly as subcutaneous tissue atrophy and hyperpigmentation, this disorder seriously affects the life quality and mental health of patients,” said the study’s first corresponding author, Xiaojun Wang, M.D., chief of the plastic surgery department at Peking Union Medical College Hospital (PUMCH). Along with Prof. Wang and her PUMCH colleagues, the study team included researchers at Jimo Traditional Chinese Medicine Hospital, Shanghai University, Chinese Academy of Medical Sciences, and the University of Oxford.
Autologous fat grafting (AFG) is currently the primary surgical treatment used to improve facial atrophy in LoS patients, but for various reasons most of the fat graft does not survive. “As a result, the patient has to undergo numerous rounds of grafting procedures to maintain their appearance, which is not only hard on them physically and mentally but poses a financial burden as well,” said the study’s first authors, Chenyu Wang, M.D., and Xiao Long, M.D.
Some studies indicate that SVF-assisted fat grafting can improve fat graft retention by about 35 percent in healthy people, but it hasn’t been proven to induce a similar increase in people with LoS. “However, ADSCs have shown potential for improving fat retention,” Prof. Long said. ADSCs, which are harvested from adult fat, have many advantages, researchers believe. They have been proven to be safe, they have self-renewal capacity and can undergo differentiation to mature cells. These cells also are very easy to propagate in cell culture, compared to other cell types.
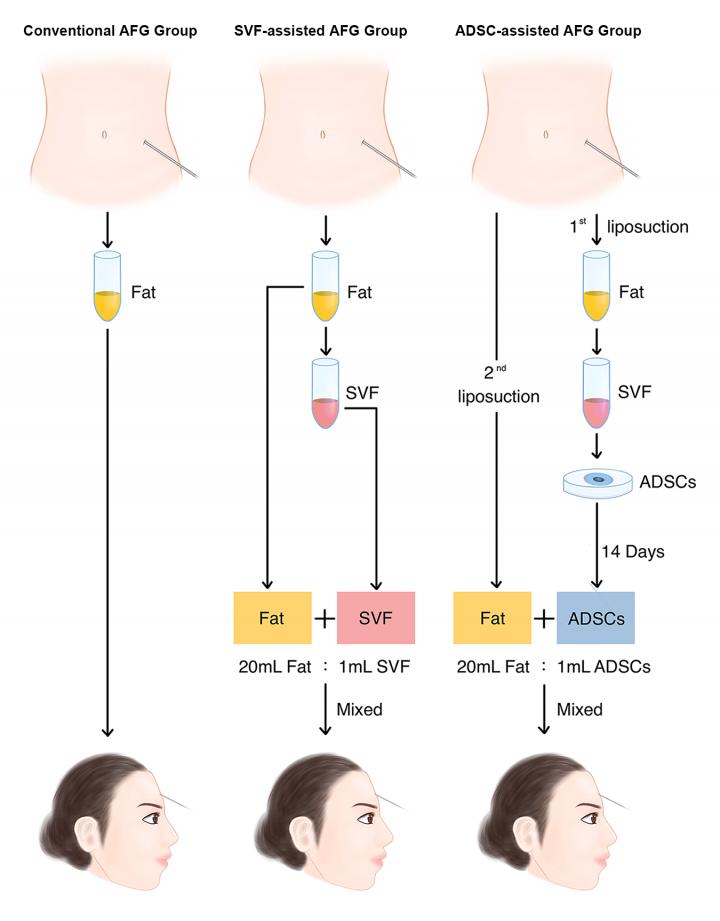
The Wang team’s study is believed to be the first clinical study employing ADSCs-assisted fat grafting to treat facial atrophy of LoS. It is also the first to perform a side-by-side comparison of these grafting therapies, revealing significant differences in graft retention. It was performed on 18 LoS patients exhibiting facial atrophy on their foreheads and cheeks. One group received conventional AFG; a second group was treated with SVF-assisted AFG; and a third group received fat grafting with ex vivo-enriched ADSCs. The mixture of ADSCs-enriched fat grafts was supplemented with 5 × 105 ADSCs/ml fat, a concentration chosen based on the protocols of previous clinical studies of ADSC transplantation.
Magnetic resonance imaging was then used to measure the facial atrophy volume of each participant preoperatively, as well as postoperatively at intervals of three and six months. Clinical photographs also were taken for outcome evaluation.
“The results showed that the fat graft retention of the ADSCs-assisted group was significantly higher than that of the SVF-assisted and the conventional fat grafting groups,” Prof. Wang said. “The ADSCs-assisted group showed notable graft retention over the rest of the groups at an early stage and exhibited the highest level of graft retention among the three groups in the long run.
“Although this pilot study was with a limited number of participants and relatively brief follow-up, it showed that the ADSCs-assisted fat grating was not only safe and well-tolerated in LoS patients, but it also may be more feasible and superior to conventional fat grafting or SVF-assisted fat grafting in improving facial atrophy,” she added.
“Longer-term, larger and controlled clinical trials will be important to confirm the efficacy of this novel cell therapy for improving fat retention in LoS patients.”
“This pilot study suggests that fat grafts combined with their stem cells potentially provide for a safe and feasible alternative to conventional treatment methods to correct facial atrophy that can occur for scleroderma patients,” said Anthony Atala, M.D., Editor-in-Chief of STEM CELLS Translational Medicine and Director of the Wake Forest Institute for Regenerative Medicine. “We look forward to the continuation of this research to further document clinical efficacy.”
###
The full article, “A pilot study on ex-vivo expanded autologous adipose-derived stem cells of improving fat retention in localized scleroderma patients,” can be accessed at https:/
About STEM CELLS Translational Medicine: STEM CELLS Translational Medicine (SCTM), co-published by AlphaMed Press and Wiley, is a monthly peer-reviewed publication dedicated to significantly advancing the clinical utilization of stem cell molecular and cellular biology. By bridging stem cell research and clinical trials, SCTM will help move applications of these critical investigations closer to accepted best practices. SCTM is the official journal partner of Regenerative Medicine Foundation.
About AlphaMed Press: Established in 1983, AlphaMed Press with offices in Durham, NC, San Francisco, CA, and Belfast, Northern Ireland, publishes two other internationally renowned peer-reviewed journals: STEM CELLS®, celebrating its 39th year, is the world’s first journal devoted to this fast paced field of research. The Oncologist®, also a monthly peer-reviewed publication, entering its 26th year, is devoted to community and hospital-based oncologists and physicians entrusted with cancer patient care. All three journals are premier periodicals with globally recognized editorial boards dedicated to advancing knowledge and education in their focused disciplines.
About Wiley: Wiley, a global company, helps people and organizations develop the skills and knowledge they need to succeed. Our online scientific, technical, medical and scholarly journals, combined with our digital learning, assessment and certification solutions, help universities, learned societies, businesses, governments and individuals increase the academic and professional impact of their work. For more than 200 years, we have delivered consistent performance to our stakeholders. The company’s website can be accessed at http://www.
About Regenerative Medicine Foundation (RMF): The non-profit Regenerative Medicine Foundation fosters strategic collaborations to accelerate the development of regenerative medicine to improve health and deliver cures. RMF pursues its mission by producing its flagship World Stem Cell Summit, honouring leaders through the Stem Cell and Regenerative Medicine Action Awards, and promoting educational initiatives.
Media Contact
Chelsea Kekahuna
[email protected]
Related Journal Article
http://dx.




