Chemists can now produce an important class of small proteins called cysteine-rich peptides in their naturally folded 3D structure more reliably and much faster, thanks to methods that mimic what happens inside cells. The advance, achieved by researchers at Xi’an Jiaotong-Liverpool University (XJTLU) in China and Nanyang Technological University (NTU) in Singapore, is published in the journal Angewandte Chemie.
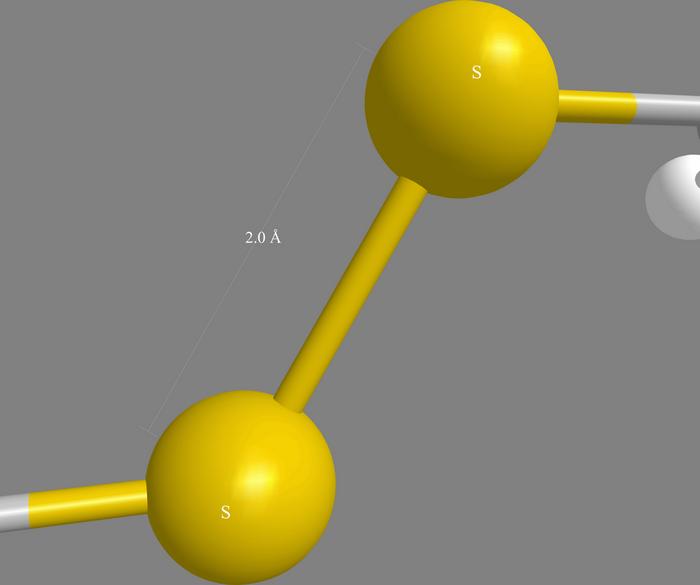
Cysteine is one of the many different amino acid molecules that can become linked together to form protein chains. Peptides are chains that are shorter than many natural proteins. Cysteine molecules each contain a sulfur atom that can become bonded to the sulfur of another cysteine elsewhere in a protein, holding different parts of the chain together.
“Re-creating the 3D shapes of cysteine-rich peptides has always been a big problem in their manufacturing,” says Dr Shining Loo of the XJTLU team. Many bioactive proteins and peptides have multiple disulfide bonds between cysteine amino acids, which are crucial for maintaining their precise 3D folded structure. Drugs like linaclotide for constipation and ziconotide for chronic pain are examples of cysteine-rich peptide drugs on the market.
“Our procedure should unlock new opportunities for drug discovery and cost-effective manufacturing of cysteine-rich microproteins and peptides as therapeutic agents,” adds researcher Dr Antony Kam of the XJTLU team.
Nature’s influence
Credit: A7Davis
Chemists can now produce an important class of small proteins called cysteine-rich peptides in their naturally folded 3D structure more reliably and much faster, thanks to methods that mimic what happens inside cells. The advance, achieved by researchers at Xi’an Jiaotong-Liverpool University (XJTLU) in China and Nanyang Technological University (NTU) in Singapore, is published in the journal Angewandte Chemie.
Cysteine is one of the many different amino acid molecules that can become linked together to form protein chains. Peptides are chains that are shorter than many natural proteins. Cysteine molecules each contain a sulfur atom that can become bonded to the sulfur of another cysteine elsewhere in a protein, holding different parts of the chain together.
“Re-creating the 3D shapes of cysteine-rich peptides has always been a big problem in their manufacturing,” says Dr Shining Loo of the XJTLU team. Many bioactive proteins and peptides have multiple disulfide bonds between cysteine amino acids, which are crucial for maintaining their precise 3D folded structure. Drugs like linaclotide for constipation and ziconotide for chronic pain are examples of cysteine-rich peptide drugs on the market.
“Our procedure should unlock new opportunities for drug discovery and cost-effective manufacturing of cysteine-rich microproteins and peptides as therapeutic agents,” adds researcher Dr Antony Kam of the XJTLU team.
Nature’s influence
Inspired by how nature quickly folds proteins inside cells, the researchers tried a different approach for the ‘oxidative’ folding reactions that form the disulfide bonds. Instead of using water-based (aqueous) solutions they used a mixture of organic solvents. This method imitates the natural enzyme that mediates the disulfide bond formation, by creating a highly reactive environment to greatly speed up the formation and rearrangement of these bonds.
By learning from nature in this way, the team was able to make 15 different peptides and microproteins, between 14 to 58 amino acids long with two to five disulfide bonds, at rates more than 100,000 times faster than could be achieved in aqueous solvents.
“The folding was efficiently completed within one second,” Dr Loo remarks, “And the range of microproteins we produced demonstrates that our method should be effective with a much larger range of peptides and microproteins in future investigations.”
This discovery is the latest advance from the XPad (XJTLU Peptide and Drug) research group, jointly established by Dr Loo and Dr Kam. This group is committed to using tools from chemical biology, synthetic biology, and molecular pharmacology to advance the application of peptides for developing therapeutic agents.
“The future of peptide research holds great promise, and we are committed to delivering even more valuable advancements in this field,” Dr Kam concludes.
Journal
Angewandte Chemie
DOI
10.1002/anie.202317789
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Ultrafast Biomimetic Oxidative Folding of Cysteine-rich Peptides and Microproteins in Organic Solvents
Article Publication Date
11-Feb-2024
COI Statement
The authors declare no conflict of interest.




