A groundbreaking study from Japan has uncovered a profound genetic mechanism through which vitamin C (VC) enhances skin regeneration, revealing promising implications for combating age-related thinning of the epidermis. Known primarily for its antioxidant capabilities and role in collagen synthesis, vitamin C is now emerging as a powerful epigenetic modulator that directly activates proliferative genes in epidermal cells, thereby stimulating skin thickening and regeneration. This novel insight stems from meticulous research employing advanced molecular biology techniques and laboratory-grown human epidermal models, demonstrating that VC promotes DNA demethylation processes essential for cellular proliferation and epidermal renewal.
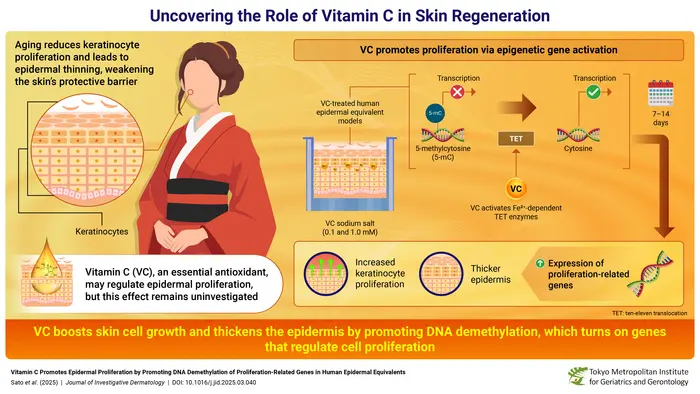
The skin’s outermost layer, the epidermis, serves as the body’s primary defensive barrier against environmental hazards, including pathogens, ultraviolet radiation, and chemical irritants. Keratinocytes, constituting approximately 90% of epidermal cells, continuously proliferate and migrate upward from basal layers to form this protective shield. However, as aging progresses, this dynamic regenerative capacity declines, resulting in epidermal thinning and compromised barrier functions. The physiological significance of this decline is profound, with thinner skin being more susceptible to injury, slower to heal, and prone to various dermatological conditions. Investigating strategies to enhance keratinocyte proliferation and restore epidermal integrity has thus become a crucial focus in dermatological research.
Vitamin C has long held a pivotal place in dermatology owing to its antioxidative properties and ability to stimulate collagen production, foundational for dermal strength and elasticity. But the molecular intricacies of how VC affects keratinocyte biology, especially through epigenetic regulation, have remained elusive until now. The Japanese research team led by Dr. Akihito Ishigami at the Tokyo Metropolitan Institute for Geriatrics and Gerontology deployed sophisticated genetic and biochemical assays on human epidermal equivalents—three-dimensional, lab-grown tissue cultures that faithfully replicate the structural and functional characteristics of natural human skin. In these models, vitamin C was administered at concentrations reflective of physiological levels transported from the bloodstream into the epidermis, enabling precise elucidation of its cellular effects.
.adsslot_YPUG8pdrMS{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_YPUG8pdrMS{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_YPUG8pdrMS{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Delving deeper into the genetic regulatory mechanisms, the researchers discovered that VC activates an epigenetic pathway centered on DNA demethylation. DNA methylation, the covalent addition of methyl groups primarily at cytosine residues within CpG dinucleotides, is a well-known repressive mark that silences gene expression. The removal of these methyl groups, or demethylation, reverses gene silencing and reactivates transcriptionally dormant genes. Specifically, vitamin C was found to sustain the catalytic activity of ten-eleven translocation (TET) enzymes, crucial epigenetic modifiers that oxidize 5-methylcytosine (5-mC) into 5-hydroxymethylcytosine (5-hmC), thereby initiating DNA demethylation.
TET enzymes require ferrous iron (Fe^2+) as a cofactor to perform oxidative demethylation reactions, but during their enzymatic cycle, Fe^2+ is oxidized to ferric iron (Fe^3+), temporarily inactivating the enzyme. Vitamin C acts as a potent reducing agent, donating electrons to convert Fe^3+ back to Fe^2+, effectively “recharging” TET enzymes and allowing sustained epigenetic activity. This regeneration cycle is vital for continuous DNA demethylation and gene activation in keratinocytes. The team’s experiments demonstrated that inhibiting TET activity abolished the VC-induced upregulation of proliferation-related genes, solidifying the centrality of TET-mediated demethylation in this regulatory axis.
Through genome-wide methylation profiling, the researchers identified over 10,000 differentially hypomethylated regions in VC-treated epidermal cells, correlating with significant upregulation—ranging from 1.6- to 75-fold—in 12 critical genes directly implicated in cell cycle progression and proliferation. This robust activation of proliferation-related genetic programs elucidates how vitamin C drives the volumetric thickening of the epidermis and enhanced keratinocyte growth. These findings not only unravel the epigenetic underpinnings of VC’s biological effects on skin but also emphasize the precision with which nutrient-derived factors can modulate gene expression landscapes.
The implications of these findings are particularly compelling in the context of aging skin. With advancing age, the epidermis loses regenerative capacity, partly due to epigenetic silencing of proliferation-essential genes. Vitamin C’s capacity to reverse such gene repression by promoting DNA demethylation suggests a therapeutic avenue to reinvigorate aged skin and ameliorate barrier dysfunction. Beyond aging, conditions characterized by epidermal thinning or compromised wound healing might benefit immensely from topical or systemic VC interventions designed to harness this epigenetic regulation pathway.
Importantly, the research underscores the elegance of the epigenome as a dynamic interface between environmental factors—like nutrient availability—and gene expression control. The reversible nature of DNA methylation positions it as an adaptive epigenetic mechanism responsive to cofactors such as vitamin C, linking metabolic state directly to cellular proliferative competence. This nexus of nutrition, epigenetics, and cell biology reflects an emerging frontier in regenerative medicine and personalized dermatology.
Moreover, the applied methodology of using human epidermal equivalents exemplifies a cutting-edge, ethical, and physiologically relevant platform to dissect complex genetic and biochemical interactions in skin tissue. Laboratory-grown skin models overcome limitations inherent in animal models and monolayer cell cultures, enabling high-resolution analysis of stratified epidermal organization and functional responses to nutrients at tissue-scale. This approach enhances confidence in translational applicability of the findings and fosters accelerated development of targeted interventions.
This landmark study not only advances fundamental understanding of epidermal biology but also paves the way for novel clinical applications that might revolutionize treatment of dermatological aging and injury. As populations worldwide face unprecedented demographic shifts toward older age groups, safe, effective, and mechanistically grounded therapies that bolster skin regeneration are increasingly critical. Vitamin C, a widely accessible and well-tolerated nutrient, now stands at the forefront of this therapeutic landscape, embodying the promise of epigenetic rejuvenation in human skin.
Subject of Research: Lab-produced tissue samples
Article Title: Vitamin C Promotes Epidermal Proliferation by Promoting DNA Demethylation of Proliferation-Related Genes in Human Epidermal Equivalents
News Publication Date: 20-Apr-2025
References:
Title of original paper: Vitamin C Promotes Epidermal Proliferation by Promoting DNA Demethylation of Proliferation-Related Genes in Human Epidermal Equivalents
Journal: Journal of Investigative Dermatology
DOI: 10.1016/j.jid.2025.03.040
Image Credits: Dr. Akihito Ishigami from Tokyo Metropolitan Institute for Geriatrics and Gerontology (TMIG), Japan
Keywords: Skin regeneration, Regeneration, Developmental biology, Life sciences, Health and medicine, Health care, Human health, Biochemistry, Nutrients, Vitamins, Vitamin C, Genetics, Molecular genetics, Human genetics, DNA demethylation, Dermatology, Medical specialties, Cell biology, Physiology
Tags: age-related skin thinningantioxidants for skin healthcollagen synthesis skin healthdermatological conditions and agingDNA demethylation in skin cellsenvironmental skin barrier protectionepidermal thickness improvementepigenetic modulation vitamin Ckeratinocyte proliferation researchmolecular biology in dermatologyregenerative medicine for skinvitamin C skin regeneration