Nucleotide excision repair (NER) is a critical DNA repair pathway that plays a key role in maintaining transcription and genome integrity by removing bulky DNA lesions.
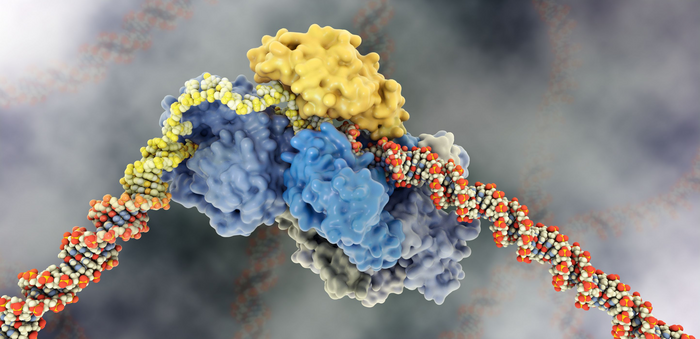
Credit: © 2022 KAUST; Heno Hwang
Nucleotide excision repair (NER) is a critical DNA repair pathway that plays a key role in maintaining transcription and genome integrity by removing bulky DNA lesions.
The key steps in the NER reaction include damage recognition, strand separation by the molecular motor TFIIH, and excision of about 30 nucleotides by the nucleases XPG and XPF to remove the damage and allow transcription to proceed without DNA damage signaling occurring. But how these steps are coordinated and regulated is not well understood.
Now, a significant advance in showing how the NER mechanism is controlled at the molecular level has been identified in a study by an international team led by researchers at KAUST and the University of Texas MD Anderson Cancer Center.
The work is potentially significant for cancer treatment, Ph.D. student and the study’s lead author Amer Bralić explains.
“During radiation therapy, cancer cells are blasted with radiation to shrink tumors. In this situation, however, NER works against the treatment, trying to repair the damage and preventing cell death, which significantly reduces the effectiveness of the treatment.”
For many years, researchers have sought a biologically safe NER inhibitor that could be given to cancer patients to increase the effectiveness of radiation treatment. However, a significant obstacle in designing inhibitors is the lack of basic knowledge about the NER mechanism. Samir Hamdan’s group at KAUST are experts in single-molecule analysis of human DNA replication and repair and have used this technique to reveal how 30 proteins mediate NER.
They have uncovered how TFIIH uses XPG to stimulate its motor activity to locate damaged DNA. In turn, once TFIIH locates the damage, it licenses the XPG nuclease activity to excise it. This significant discovery was selected as a “breakthrough article” by the journal Nucleic Acids Research. “The finding unravels a fundamental control mechanism in NER and argues for tackling the interaction between TFIIH and XPG as an effective drug target,” says Hamdan.
A complex mutation landscape in NER proteins mediates more than 10 clinical diseases, where mutations in one protein may cause different diseases and different combinations of proteins may cause one disease. “Our mechanistic findings provide new perspectives on linking molecular level information to disease states,” explains Bralić.
“Through the KAUST Smart Health Initiative, we will work with clinicians in the Kingdom to study the clinical mutational landscape of NER proteins in patients,” concludes Hamdan.
Journal
Nucleic Acids Research
DOI
10.1093/nar/gkac1095
Article Title
A scanning-to-incision switch in TFIIH-XPG induced by DNA damage licenses nucleotide excision repair
Article Publication Date
8-Dec-2022




