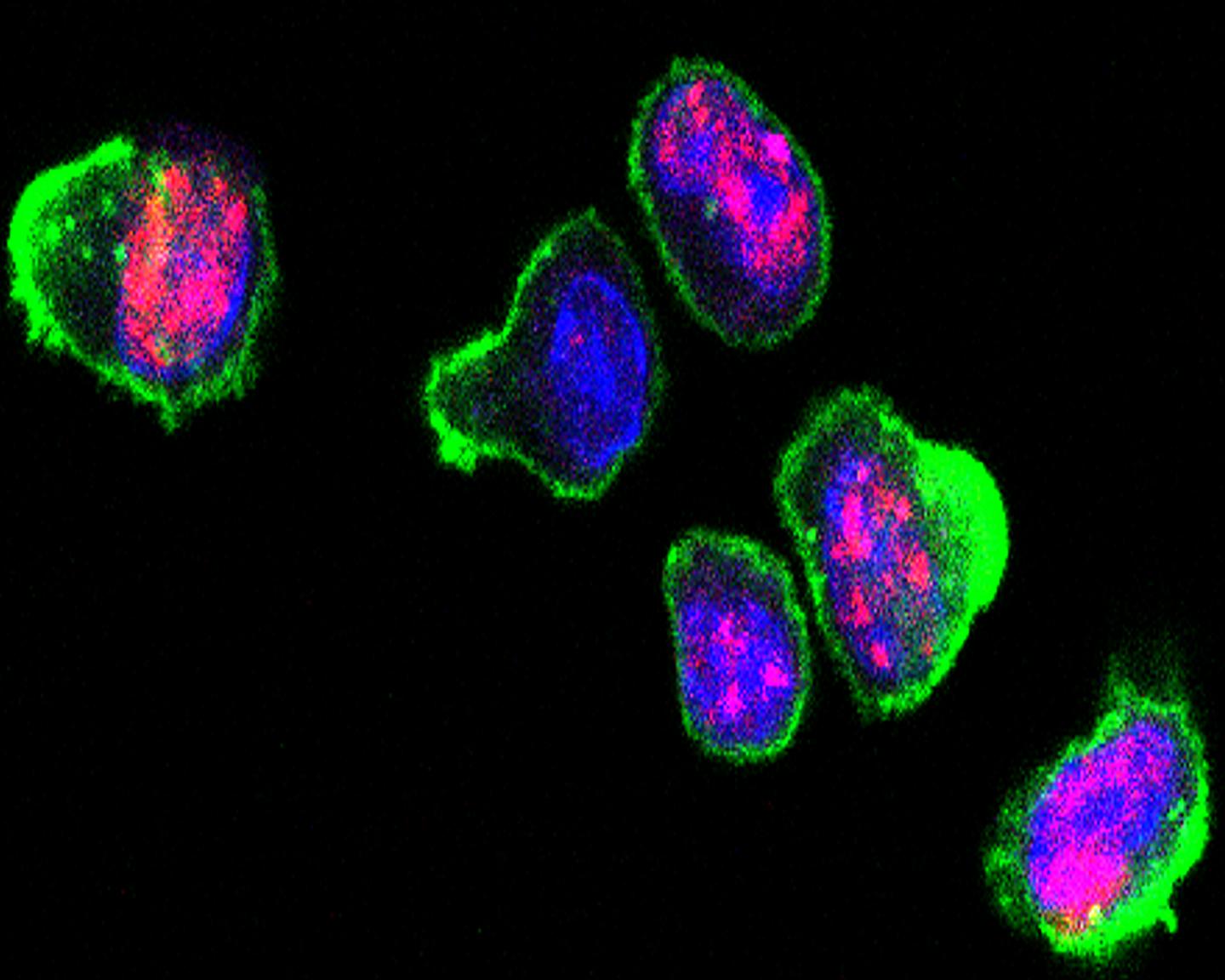
Credit: Cincinnati Children's
CINCINNATI – Immune diseases like multiple sclerosis and hemophagocytic lymphohistiocytosis unleash destructive waves of inflammation on the body, causing death or a lifetime of illness and physical impairment. With safe and effective treatments in short supply, scientists report in PNAS Early Edition (Proceeding of the National Academy of Sciences) discovery of an experimental treatment that targets an Achilles heel of activated immune cells – killing them off and stopping autoimmune damage.
Researchers at Cincinnati Children's Hospital Medical Center report in a study published the week of May 22 that a treatment modality they call PPCA takes advantage of DNA damage in rapidly expanding T cells, which they show was therapeutically beneficial in mouse models of hemophagocytic lymphohistiocytosis (HLH) and multiple sclerosis (MS). And for the most part it appears to be so without harming other immune system components needed to protect the body from infection.
"We found that when T cells activate and go through extraordinarily rapid cell division during initial immune responses, it leads to an unusual level of genomic stress in the cells," explains Michael B. Jordan, MD, lead author and physician/scientist in the divisions of Bone Marrow Transplantation and Immune Deficiency and Immunobiology.
"Because T cells are always in a race with different viruses and bacteria, they have learned how to adapt and divide rapidly to respond, but this stress on their DNA means they also are living right on the edge of death," Jordan says. "In our experiments we selectively interrupted DNA damage repair in rapidly expanding T cells, and we threw them off balance and into a chasm of death."
Harnessing a Guardian Angel
PPCA is a newly minted acronym for "p53 potentiation with checkpoint abrogation." The therapeutic approach was developed by Jordan and his colleagues, including Jonathan Katz, PhD, and David Hildeman, PhD, (Division of Immunobiology). It was conceived during experiments on mouse and donated human immune cells called lymphocytes, which include the aggressively effective germ killers, T cells and B cells.
Researchers hypothesized that along with the highly adaptable and proliferative abilities of T cells came an abundance of genomic stress. They observed as T cells used DNA damage response pathways to survive while adapting and gearing up to attack lymphocytic choriomeningitis virus (LCMV) as it tried to infect cells and animal models.
A gene and its protein called p53 (also called the "guardian of the genome") helps initiate DNA damage repair – the primary reason researchers decided to target it in T cells. They also leveraged a concept developed for the treatment of cancer called cell cycle checkpoint inhibition or abrogation – in which cells are forced to lose normal control over the mitotic cell division cycle.
Precise Targeting
In selective instances of rapid T cell expansion in mouse models of HLH and experimental autoimmune encephalomyelitis (experimental mouse MS), the researchers used a small molecule called Nutlin to alter the activities of p53. They also inhibited cell cycle checkpoint proteins known as CHK1/2 or WEE1. This prevented the T cells from pausing and repairing their DNA damage, which prompted them to die off.
In mouse models of HLH – mainly a childhood disease where the immune system overheats, attacks healthy tissues, damages organs and causes early death – PPCA reduced disease in the animals and allowed them to survive long-term.
The researchers also tested PPCA treatment in mice with experimental autoimmune encephalomyelitis (EAE) used to model multiple sclerosis. In MS, autoimmune-driven inflammation damages a protective insulating sheath on nerves called myelin. This causes disruptions in the central nervous system that disrupt signals between the brain and extremities, which can lead to paralysis and other symptoms.
In EAE mouse models of MS, PPCA treatment killed off aggressively expanding T cells, tempered autoimmune processes and either reversed or prevented paralysis in the animals, authors report in their study.
Future Steps
Jordan and his research colleagues – including first author Jonathan P. McNally (a recently graduated PhD student in the Immunology graduate program) – caution that their experimental results are early. Years of additional research are needed before knowing whether the current findings will eventually apply to clinical treatment in humans.
The authors now plan to test PPCA in laboratory models of other autoimmune disorders to see how widely applicable it might be. Jordan is listed as an inventor of PPCA in a U.S. patent filing through the Center for Technology Commercialization at Cincinnati Children's.
###
Funding support for the research came in part from the National Institutes of Health (RO1DK081175, RO1AI109810, RO1AI057753 and a Research Innovation Grant from Cincinnati Children's.
Media Contact
Nick Miller
[email protected]
513-803-6035
@CincyChildrens
http://www.cincinnatichildrens.org
Related Journal Article
http://dx.doi.org/10.1073/pnas.1703683114