Credit: Panpan Xu
A new process for restoring spent cathodes to mint condition could make it more economical to recycle lithium-ion batteries. The process, developed by nanoengineers at the University of California San Diego, is more environmentally friendly than today’s methods; it uses greener ingredients, consumes 80 to 90% less energy, and emits about 75% less greenhouse gases.
Researchers detail their work in a paper published Nov 12 in Joule.
The process works particularly well on cathodes made from lithium iron phosphate, or LFP. Batteries made with LFP cathodes are less costly than other lithium-ion batteries because they don’t use expensive metals like cobalt or nickel. LFP batteries also have longer lifetimes and are safer. They are widely used in power tools, electric buses and energy grids. They are also the battery of choice for Tesla’s Model 3.
“Given these advantages, LFP batteries will have a competitive edge over other lithium-ion batteries in the market,” said Zheng Chen, a professor of nanoengineering at UC San Diego.
The problem? “It’s not cost-effective to recycle them,” Chen said. “It’s the same dilemma with plastics–the materials are cheap, but the methods to recover them are not.”
The new recycling process that Chen and his team developed could lower these costs. It does the job at low temperatures (60 to 80 C) and ambient pressure, making it less power hungry than other methods. Also, the chemicals it uses–lithium salt, nitrogen, water and citric acid–are inexpensive and benign.
“The whole regeneration process works at very safe conditions, so we don’t need any special safety precautions or special equipment. That’s why we can make this so low cost for recycling batteries,” said first author Panpan Xu, a postdoctoral researcher in Chen’s lab.
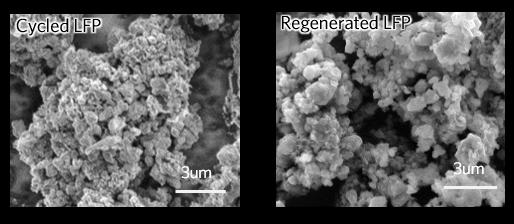
The researchers first cycled commercial LFP cells until they had lost half their energy storage capacity. They took the cells apart, collected the cathode powders, and soaked them in a solution containing lithium salt and citric acid. Then they washed the solution with water, dried the powders and heated them.
The researchers made new cathodes from the powders and tested them in both coin cells and pouch cells. Their electrochemical performance, chemical makeup and structure were all fully restored to their original states.
As the battery cycles, the cathode undergoes two main structural changes that are responsible for its decline in performance. The first is the loss of lithium ions, which creates empty sites called vacancies in the cathode structure. The other occurs when iron and lithium ions switch spots in the crystal structure. When this happens, they cannot easily switch back, so lithium ions become trapped and can no longer cycle through the battery.
The process restores the cathode’s structure by replenishing lithium ions and making it easy for iron and lithium ions to switch back to their original spots. The latter is accomplished using citric acid, which acts as a reducing agent–a substance that donates an electron to another substance. Citric acid transfers electrons to the iron ions, making them less positively charged. This minimizes the electronic repulsion forces that prevent the iron ions from moving back into their original spots in the crystal structure, and also releases the lithium ions back into circulation.
While the overall energy costs of this recycling process are lower, researchers say further studies are needed on the logistics of collecting, transporting and handling large quantities of batteries.
“Figuring out how to optimize these logistics is the next challenge,” Chen said. “And that will bring this recycling process closer to industry adoption.”
###
Media Contact
Liezel Labios
[email protected]
Related Journal Article
http://dx.




