Photoluminescent molecules, capable of absorbing and re-emitting light, play an important role in the development of technologies such as light-emitting diodes, sensors, and displays. Among them, ordered arrangements of π-electronic molecules such as crystals of organoplatinum(II) complexes, where a platinum(II) ion is coordinated by organic ligands in a square-planar arrangement, stand out for their applications in energy-efficient flexible displays. However, their luminescence in the solid state is short-lived due to the interaction between excitons (bound electron-hole pairs) of neighboring molecules. To address this issue, bulky foreign molecules are introduced into the molecular structure to prevent or minimize the electronic interactions between molecules.
Credit: Hiromitsu Maeda from Ritsumeikan University
Photoluminescent molecules, capable of absorbing and re-emitting light, play an important role in the development of technologies such as light-emitting diodes, sensors, and displays. Among them, ordered arrangements of π-electronic molecules such as crystals of organoplatinum(II) complexes, where a platinum(II) ion is coordinated by organic ligands in a square-planar arrangement, stand out for their applications in energy-efficient flexible displays. However, their luminescence in the solid state is short-lived due to the interaction between excitons (bound electron-hole pairs) of neighboring molecules. To address this issue, bulky foreign molecules are introduced into the molecular structure to prevent or minimize the electronic interactions between molecules.
Using this strategy, a research team led by Professor Hiromitsu Maeda from Ritsumeikan University, Japan, recently enhanced the solid-state phosphorescence in multiple organoplatinum(II) complexes, increasing the phosphorescence by upto 75 times. “Spatially and electronically isolated ordered arrangement of emissive π-electronic molecules is a principal point for the preparation of emissive solid-state materials. This concept can be used in materials for organic electronics, particularly organic light-emitting diodes for flexible displays,” explains Prof. Maeda.
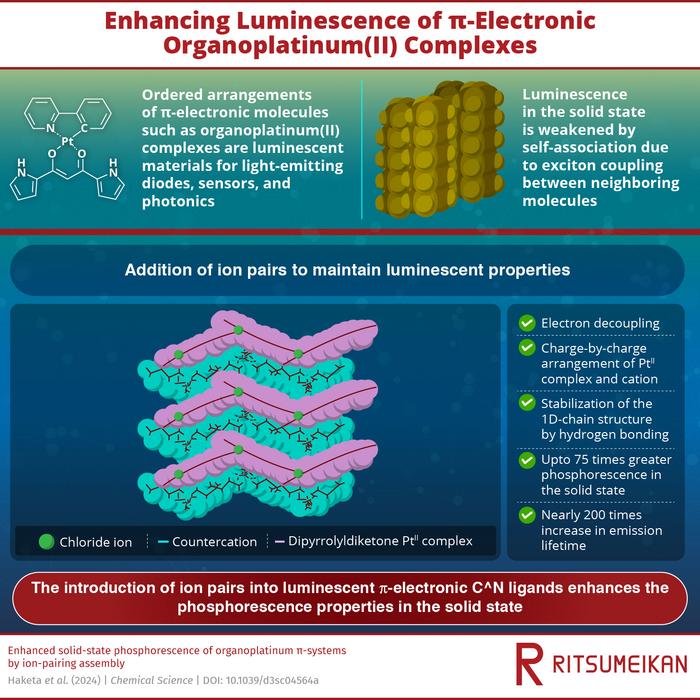
In their study published in Chemical Science on December 5, 2023, the research team synthesized dipyrrolyldiketone PtII complexes consisting of four different C^N ligands. These molecules display strong phosphorescence in solution but show extremely weak phosphorescence in the solid state due to self-association. To enhance their luminosity in the solid state, the team introduced ion pairs consisting of a chloride anion and tetraalkylammonium countercations: TPA+ (tetrapropylammonium), TBA+ (tetrabutylammonium), and TPeA+ (tetrapentylammonium). This resulted in ion-pairing assemblies consisting of chloride ion-binding PtII complexes and countercations. The chloride ions bind to the PtII complex via hydrogen bonds, while the cations form layers between the π-electronic molecules. X-ray analysis confirmed the complex’s rigid structure, where PtII complexes are separated by cations in charge-by-charge arrangements.
By isolating the π-electronic molecules from each other, the researchers enhanced the luminescent properties of the organoplatinum(II) complexes in the solid state. Compared to the original anion-free states where the complex is not bonded to the chloride ion, the relative intensity of phosphorescence in Cl−-binding PtII complexes with cations showed improvements ranging from 1% to 7.5%, a 75-fold increase over the original molecule. The luminescence also lasts significantly longer, with certain ion-pairing assemblies achieving an emission lifetime nearly 200 times longer than the monomeric PtII complex. Theoretical studies using DFT calculations revealed that the charge-by-charge packing structure prevents the delocalization of the electron wavefunction over PtII complexes.
“To the best of our knowledge, such a room-temperature phosphorescence enhancement by anion binding and ion-pairing assembly has not been demonstrated thus far,” remarks Prof. Maeda.
Such a strategy can be used to design emissive materials and improve the phosphorescence of solid-state materials for novel applications. “The chemistry of ion-pairing assembly of charged π-electronic molecules is a new topic in a research area of supramolecular chemistry. Understanding the interactions between charged species and the formation of assembled structures through research will affect in a further design and fabrication of functional ion-pairing assemblies such as efficient electric conductive materials, ferroelectric materials, and chiral transfer in ion pair and the ion-pairing assemblies exhibiting fascinating optical properties,” concludes Prof. Maeda.
***
Reference
DOI: https://doi.org/10.1039/d3sc04564a
About Ritsumeikan University, Japan
Ritsumeikan University is one of the most prestigious private universities in Japan. Its main campus is in Kyoto, where inspiring settings await researchers. With an unwavering objective to generate social symbiotic values and emergent talents, it aims to emerge as a next-generation research university. It will enhance researcher potential by providing support best suited to the needs of young and leading researchers, according to their career stage. Ritsumeikan University also endeavors to build a global research network as a “knowledge node” and disseminate achievements internationally, thereby contributing to the resolution of social/humanistic issues through interdisciplinary research and social implementation.
Website: http://en.ritsumei.ac.jp/
Ritsumeikan University Research Report: https://www.ritsumei.ac.jp/research/radiant/eng/
About Professor Hiromitsu Maeda from Ritsumeikan University, Japan
Hiromitsu Maeda is a professor at the Department of Applied Chemistry, College of Life Sciences, Ritsumeikan University. He completed his PhD from Kyoto University in 2004. Professor Maeda’s research interests include topics like physical organic chemistry, supramolecular chemistry, and materials science on π-electronic systems. Prof. Maeda has received several prizes, including the ChemComm Emerging Investigator Lectureship (2012) and Fellow of the RSC (2015), and has over 200 publications.
Funding Information
This work was supported by JSPS KAKENHI Grant Numbers JP18H01968 and JP22H02067 for Scientific Research (B), JP19K05444 and JP22K05253 for Scientific Research (C), JP20J22745 for JSPS Fellows and JP20H05863 for Transformative Research Areas (A) “Condensed Conjugation” and the Ritsumeikan Global Innovation Research Organization (R-GIRO) project (2017–22 and 2022–27)
Journal
Chemical Science
DOI
10.1039/d3sc04564a
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Enhanced solid-state phosphorescence of organoplatinum π-systems by ion-pairing assembly
Article Publication Date
5-Dec-2023
COI Statement
There are no conflicts of interest to declare




