Electron transfer is an elementary process in which an electron is transferred or relocated from a donor molecule or atom to another such entity, called the acceptor. This process is fundamental to chemical reactions, electronic devices, and even living organisms. Understanding electron transfer steps in a solid/solid interface is important for improving the performance of organic optoelectronic devices, such as organic light emitting diodes (OLEDs) and organic photovoltaics, which have wide applications in digital displays and portable electronics due to their lightweight and flexible structure.
Electron transfer is an elementary process in which an electron is transferred or relocated from a donor molecule or atom to another such entity, called the acceptor. This process is fundamental to chemical reactions, electronic devices, and even living organisms. Understanding electron transfer steps in a solid/solid interface is important for improving the performance of organic optoelectronic devices, such as organic light emitting diodes (OLEDs) and organic photovoltaics, which have wide applications in digital displays and portable electronics due to their lightweight and flexible structure.
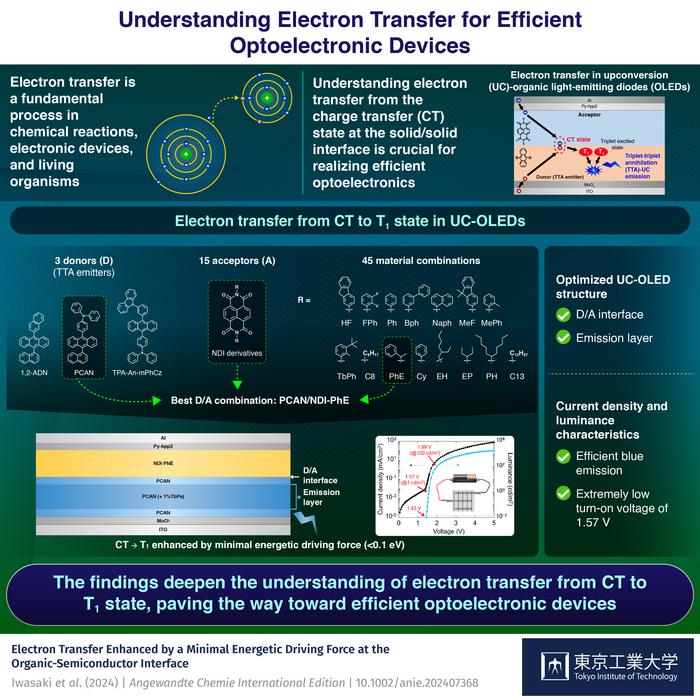
A key intermediate step in the operation of these devices involves the charge transfer (CT) state, which is a weakly bound electron-hole pair at the donor/acceptor interface. To enhance the performance of these devices, it is important to understand the energetic and structural factors of electron transfer steps, which can be achieved by experimentally studying electron transfer reactions from the CT state to other excited states using the Marcus theory. This theory explains electron transfer according to the energetics and structures. However, such analyses of electron transfer have been limited owing to various influencing factors. Additionally, recent studies have proposed the concept of upconversion OLEDs (UC-OLEDs) which utilize electron transfer from the CT state to a triplet excited (T1) state, through a mechanism called triplet-triplet annihilation (TTA). This mechanism can significantly lower the turn-on voltage of blue UC-OLEDs compared to conventional blue OLEDs, potentially addressing issues like high driving voltage and low stability.
To address this knowledge gap, a team of researchers from Japan, led by Associate Professor Seiichiro Izawa from the Laboratory for Materials and Structures at Tokyo Institute of Technology investigated electron transfer efficiency from the CT state to the T1 state of the donor molecule in 45 UC-OLEDs. Dr. Izawa explains, “Unlike common organic molecules, UC-OLEDs show distinct CT and TTA emissions at different wavelengths without overlap, making them ideal for simultaneously analyzing device efficiency and the electron transfer between CT and T1 states. These analyses can not only lead to efficient blue UC-OLEDs but can also enhance our understanding of other such devices.” Their findings were published in the journal Angewandte Chemie International Edition on June 24, 2024.
The researchers systematically analyzed different UC-OLEDs using 45 different material combinations of three anthracene derivatives as donors, and 15 naphthalenediimide derivatives as acceptors, guided by the Marcus theory. Their analysis revealed that the electron transfer from the CT state to the T1 state is enhanced by a minimal driving energetic force, less than 0.1 eV. They also discovered a novel donor-acceptor combination, PCAN/NDI-PhE, from the Marcus plots, which they used to fabricate an efficient blue UC-OLED with an extremely low turn-on voltage of 1.57 V.
“Our findings deepen the understanding of the mechanisms underlying the electron transfer from the CT state to T1, leading to the more efficient UC-OLEDs,” remarks Dr. Izawa, emphasizing the importance of the study. Looking towards the future, he concludes, “We believe that precise control over energetics and structural factors of the donor/acceptor interface will significantly enhance the electron transfer, paving the way for highly efficient organic optoelectronic devices without energy loss.”
###
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
https://www.titech.ac.jp/english/
Institute of Science Tokyo (Science Tokyo) will be established on October 1, 2024, following the merger between Tokyo Medical and Dental University (TMDU) and Tokyo Institute of Technology (Tokyo Tech), with the mission of “Advancing science and human wellbeing to create value for and with society.”
https://www.isct.ac.jp/en
Journal
Angewandte Chemie International Edition
DOI
10.1002/anie.202407368
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Electron Transfer Enhanced by a Minimal Energetic Driving Force at the Organic-Semiconductor Interface
Article Publication Date
24-Jun-2024