Unique Rice platform helps bioscientists learn how ectoderm cells begin to differentiate
Credit: Warmflash Lab/Rice University
HOUSTON – (Oct. 17, 2019) – During embryonic development, the entire nervous system, the skin and the sensory organs emerge from a single sheet of cells known as the ectoderm. While there have been extensive studies of how this sheet forms all these derivatives, it hasn’t been possible to study the process in humans – until now.
Rice bioscientist Aryeh Warmflash, graduate student George Britton and their colleagues have created a system in which all of the major cell types of ectoderm are formed in a culture dish in a pattern similar to that seen in embryos.
This technique, based on controlling the geometry of stem cell colonies with microscale patterns, has helped them make the most comprehensive analysis yet of signaling pathways that drive patterning of human ectoderm.
“There are very few possible signals the embryo uses to generate the wide variety of cell types that arise,” Britton said. “We want to understand the timing of these signals and how the cells interpret them in time to generate this variety.”
Their study was published as a highlighted article in Development, which also published a lengthy interview with the researchers.
It revealed that the balance between two signaling pathways, BMP and Wnt, are both critical, and even a bit adaptable as they orchestrate patterning in the ectoderm. The logic they employ ultimately drives ectodermal cells to their fates, but the research showed they can take more than one road to get there.
Britton said the micropatterned plates and a better understanding of how the signaling pathways work let them manipulate stem cell colonies to form unusual patterns at the start, but ultimately they always seemed to converge at the same place. “We found different trajectories of the signals that arrived at the same pattern,” he said.
That suggested the system by which stem cells become neurons, neural crest cells, neurogenic placodes and epidermis cells is pretty robust.
“A lot of people are interested in the transcription factor network that directs neural crest emergence, so this is a powerful system to dissect the signals that contribute to that logic,” Britton said. “That was one thing we feel we contributed to the field.
“There’s also the idea that cells that have the ability to interpret relative levels of BMP and Wnt to incorporate the appropriate fate decision,” he said. “In the embryo, cells are moving around quite a bit in a space where signals and the ligands they’re exposed to are also moving around. It might be that cells are reading the relative levels to determine a certain fate.”
The researchers observed that the relative activity of BMP and Wnt signaling determines cells’ decisions to become either neural crest or placodal cells, while BMP alone initiates and controls the size of the surface ectoderm, all within about the first four days.
“Four days is about right in the sense that cells are starting to make decisions: ‘I’m going to be a placodal cell, I’m going to be a neural crest cell, I’m going to be neural fate and I’m going to an epidermal fate,” Britton said.
“We see that approximately a day or two after BMP treatment. But it’s hard to put a finger on whether these are the final patterns,” he said. “We’d have to do a more careful observation to make sure those placodal cells don’t change to neural crest cells, or vice versa. That will give us information on how these lineages and fates settle into a final pattern, maybe by day 6 or 7.”
He said future studies will further refine their understanding of how signaling patterns work, as well as how the development of all the germ layers collaborate.
“Until now, studies of human stem cells differentiating to ectodermal fates were mostly about how to get all the cells in your culture dish to become a particular cell type; for example, how to make a dish full of neurons,” Warmflash said. “We are interested in a different question: How do cells interact with each other to make patterns of different cell fates? The system we developed does this outside the embryo and is allowing us to begin to tackle this question.”
###
Co-authors of the paper are Rice alumni Idse Heemskerk, now at the University of Michigan Medical School, and Rachel Hodge, now at UCLA; and Amina Qutub, an associate professor of biomedical engineering at the University of Texas at San Antonio. Warmflash is an assistant professor of biosciences and bioengineering.
The National Institutes of Health, through the National Institute of General Medical Sciences; the National Science Foundation, the Cancer Prevention and Research Institute of Texas, the University of Texas STARS program and the Simons Foundation supported the research.
Read the abstract at https:/
This news release can be found online at https:/
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Embryos’ signals take multiple paths: http://news.
Embryos’ signaling proteins go with the flow: http://news.
Fed grant will help unlock embryonic secrets: http://news.
Warmflash lab: https:/
Department of Biosciences: https:/
Wiess School of Natural Sciences: https:/
Images for download:
https:/
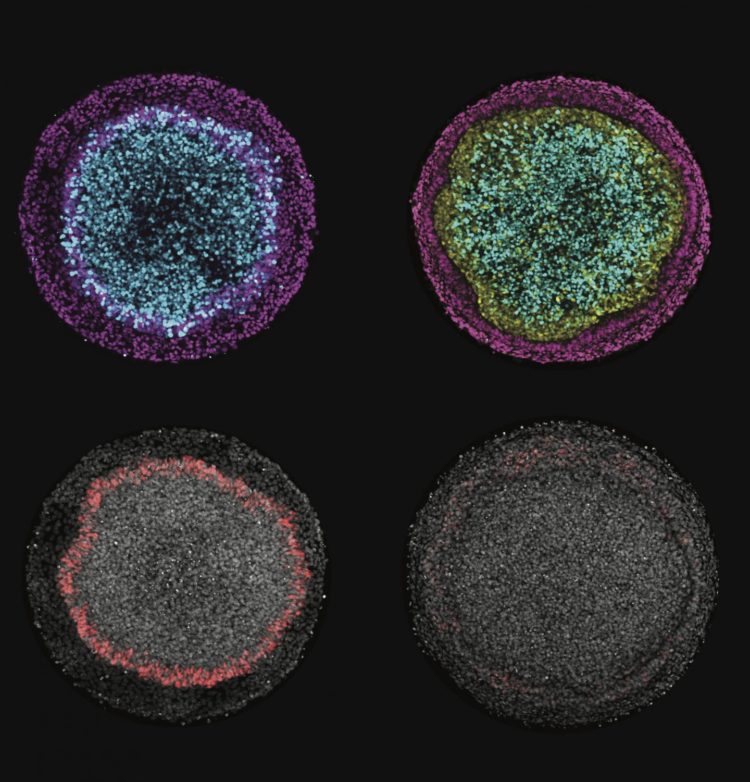
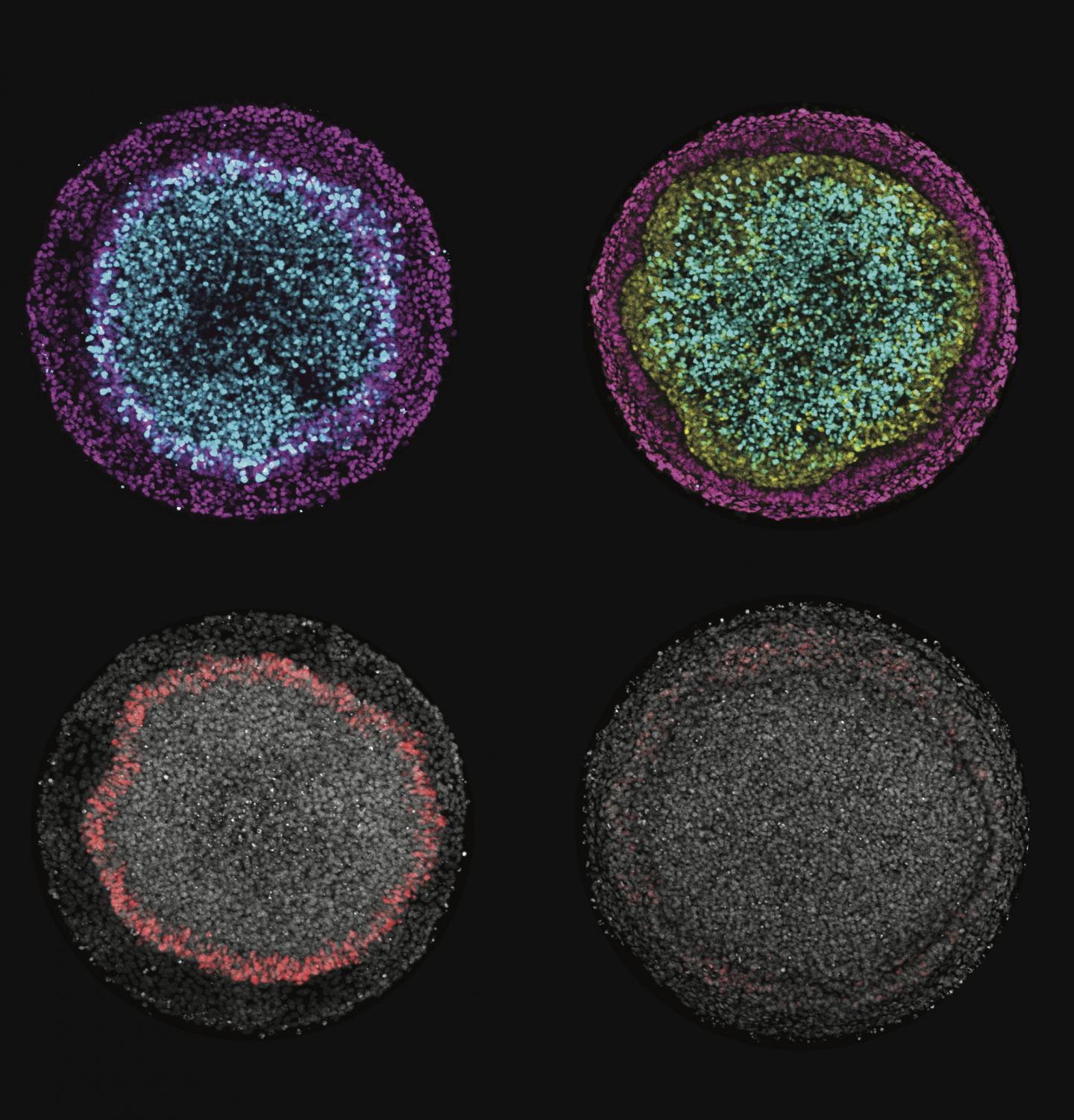
Images produced by Rice University scientists illustrate changes in ectodermal fate composition as a result of controlling the duration of endogenous Wnt secretion and signaling. Inhibition of Wnt prior to, or at the time of, BMP4 treatment instruct colonies (left column) to form radial patterns of neural (cyan), surface ectoderm (magenta) and placodal (red) fates, while permitting secondary Wnt secretion instructs colonies (right column) to form a ring of neural crest (yellow) at the expense of placodal fates. (Credit: Warmflash Lab/Rice University)
https:/
Rice University graduate student George Britton checks embryonic cell colonies in assays that employ micropatterned plates to manipulate their development. Britton and his mentor, Rice bioscientist Aryeh Warmflash, are interested in the cell patterning that takes place in embryos during early development. (Credit: Jeff Fitlow/Rice University)
https:/
Rice University graduate student George Britton checks a set of cell colonies. He and Rice bioscientist Aryeh Warmflash have created lab tools and procedures that allow them to view in fine detail the signaling pathways and processes by which early embryos form germ layers. (Credit: Jeff Fitlow/Rice University)
https:/
Micropatterned plates contain multiple colonies of embryonic cells. The plates developed at Rice University allow researchers to view and manipulate the colonies as they grow to determine how signaling pathways help cells differentiate. (Credit: Jeff Fitlow/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Jeff Falk
713-348-6775
[email protected]
Mike Williams
713-348-6728
[email protected]
Media Contact
Mike Williams
[email protected]
713-348-6728
Original Source
https:/
Related Journal Article
http://dx.